Primary Malignant Fibrous Histiocytoma (MFH-T) and Leiomyosarcoma (LMS-T) of the thyroid gland are extremely rare tumors. Very few cases have been reported in the literature. Both entities occur more commonly in women than men. The closest clinical and histological differential diagnosis is anaplastic carcinoma of thyroid.
We present three cases of rare primary sarcomas of thyroid gland. Case-1 was a 63-year-old woman and Case-2 was a 52-year-old woman. Both of them presented with a rapidly increasing thyroid mass clinically mimicking anaplastic carcinoma (AC-T). Both the patients developed pulmonary metastasis and succumbed to the illness soon after the diagnosis of MFH-T was made. Case 3 was 65-year-old woman with neck swelling since six months diagnosed as LMS-T.
The present communication adds three new cases to the literature on sarcomas of thyroid gland with an emphasis on differential diagnosis of spindle cell lesions of thyroid. MFH-T and LMS-T needs to be differentiated from AC-T, metastatic sarcomas, spindle cell variant of medullary carcinoma, synovial sarcoma, fibrosarcoma; final diagnosis rests on histopathology and immunohistochemistry.
Case Series
Case 1
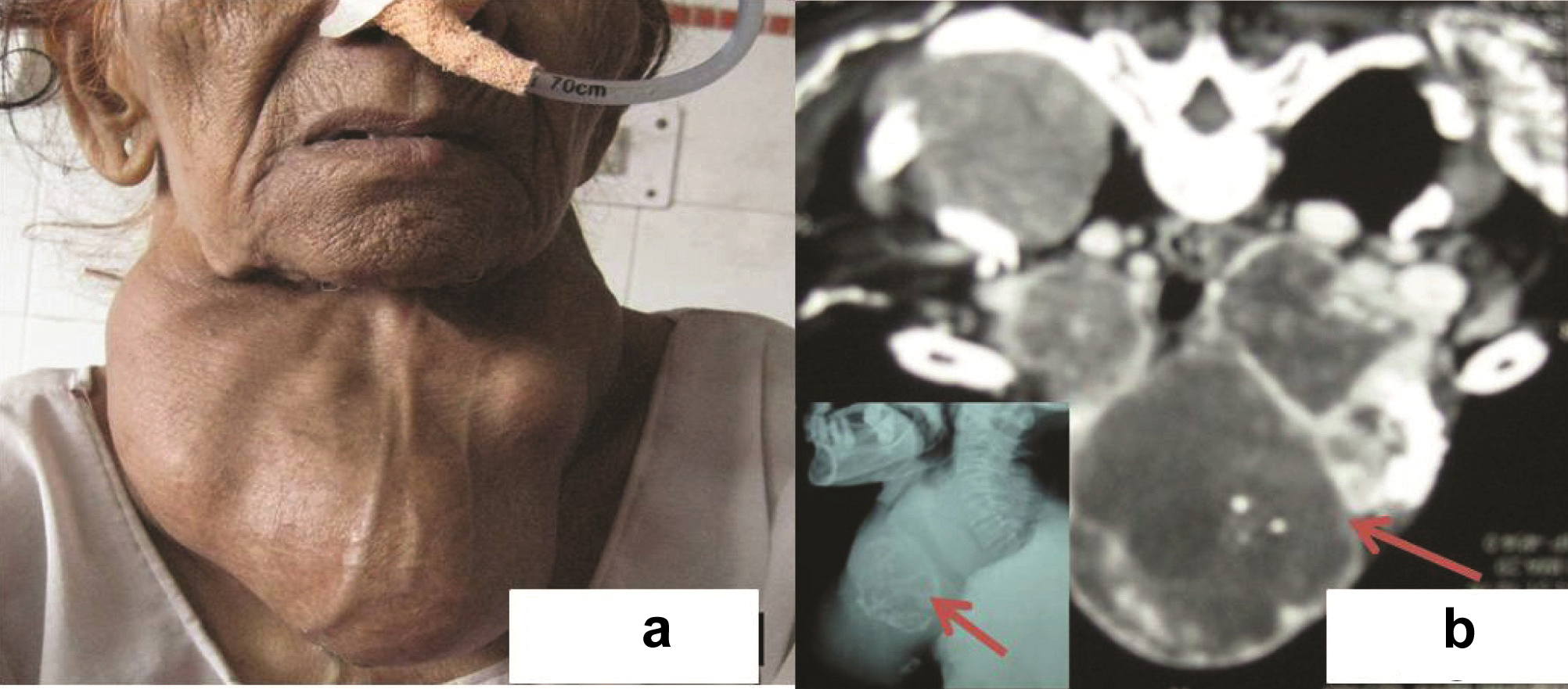
A 63-year-old woman presented with symptoms of neck constriction and rapidly growing midline neck mass for past 6 months. She was a known case of Grave’s disease and was on propylthiouracil and propranolol for past 7 years. Thyroid function tests showed free thyroxine (FT4): 1.0 ng/dl (normal limit 0.8-2.0); triiodothyroxine: 98.02 ng/dl (normal 86-187), thyrotropin (TSH): 0.32 μU/l (normal 0.3-5). Thyrotropin receptor antibody: 2.8 U/l (normal limit, <1.0); and thyroglobulin: 725 ng/ml (normal limit, 1-25). On examination, there was a large midline neck swelling measuring 14x10 cm which was fixed to underlying structures [Table/Fig-1a]. Contrast Enhanced Computed Tomography (CECT) [Table/Fig-1b] and neck X-ray ([Table/Fig-1b], inset) delineated the origin from thyroid gland, its extension into the surrounding structures, strap muscles and compressing the trachea. Fine needle aspiration from neck mass was performed which yielded haemorrhagic aspirate. Giemsa stained smears showed a hypercellular lesion. The cells were spindle shaped arranged in loose clusters with moderate degree of atypia, hyperchromatic nucleus and 1-2 prominent nucleoli [Table/Fig-2a]. Focally necrosis was also seen. Normal thyroid follicles were not seen. Papillary fragments, intranuclear inclusions, microfollicles or amyloid were not identified even after extensive search. Immunocytochemistry performed for thyroglobulin and calcitonin was non-contributory. Hence, possibility of a high grade malignant spindle cell lesion was suggested and excision of neck mass was advised. Per-operatively the tumor involved the facial planes however infiltration into surrounding structures was not found.
(a) Case 1: Old lady with a huge neck mass. (b) Neck CT: thyroid mass with necrosis and calcification compressing trachea (Arrow). Inset: Neck X-ray showing similar changes. (Arrow)
(a) Case 1-FNAC smears show spindle shaped cells with moderate degree of atypia, hyperchromatic nucleus and 1-2 prominent nucleoli. [Giemsa, x200] (b) Gross: Tumor is haemorrhagic with areas of congestion. Inset: Cut section: grey white with necrotic and haemorrhagic areas. (c) Diffuse sheets of spindle cells; normal thyroid follicles were identified interspersed between the tumor cells (arrow). (H&E, x200) Inset: An intact capsule was identified around the tumor mass; cells arranged in a vague storiform pattern. (H&E x100).
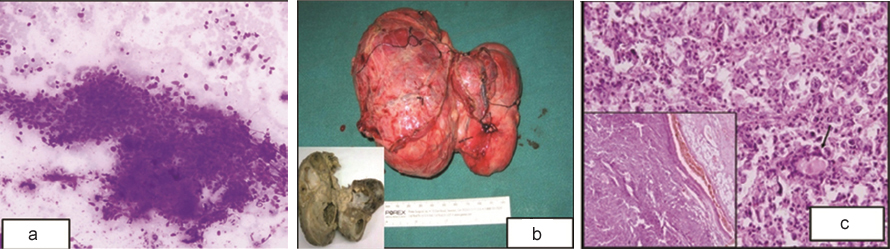
Grossly the tumor measured 13x9x5 cm [Table/Fig-2b], cut section was solid grey white with large cystic, necrotic and haemorrhagic areas ([Table/Fig-2b], inset). Normal thyroid tissue was not apparent grossly. Microscopically the tumor was encapsulated ([Table/Fig-2c], inset) and composed of diffuse sheets of spindle cells arranged in a vague storiform pattern which led us to a differential of spindle cell variant of medullary carcinoma, anaplastic carcinoma thyroid, metastatic sarcomas, synovial sarcoma or fibrosarcoma. Normal thyroid follicles were identified focally interspersed in between the tumor cells [Table/Fig-2c] with compressed and infiltrated thyroid tissue. Individual tumor cells were polygonal to spindled, highly pleomorphic with many multinucleated tumor giant cells along with cells with bizarre hyperchromatic nuclei and irregular macronucleoli.
Case 2
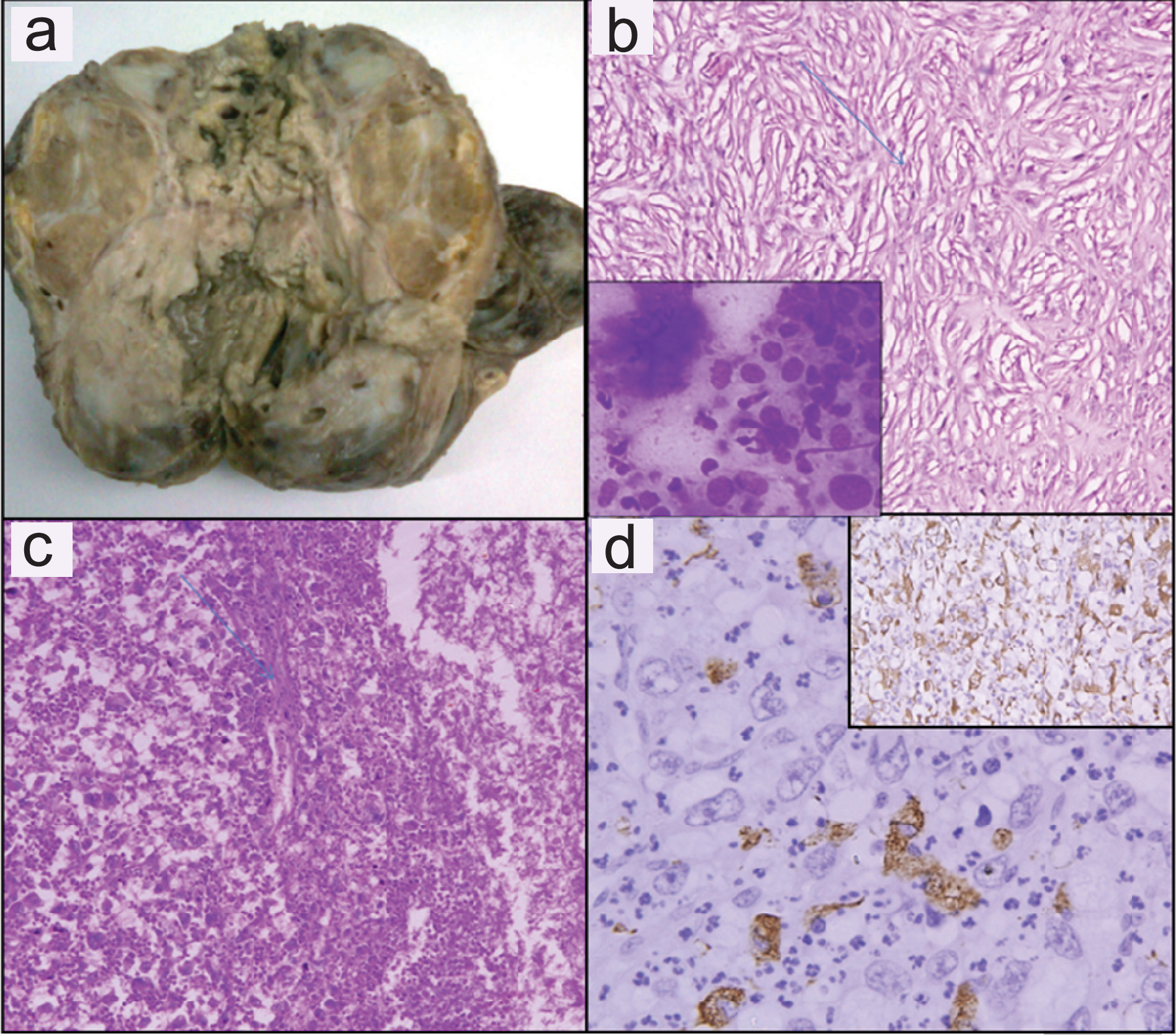
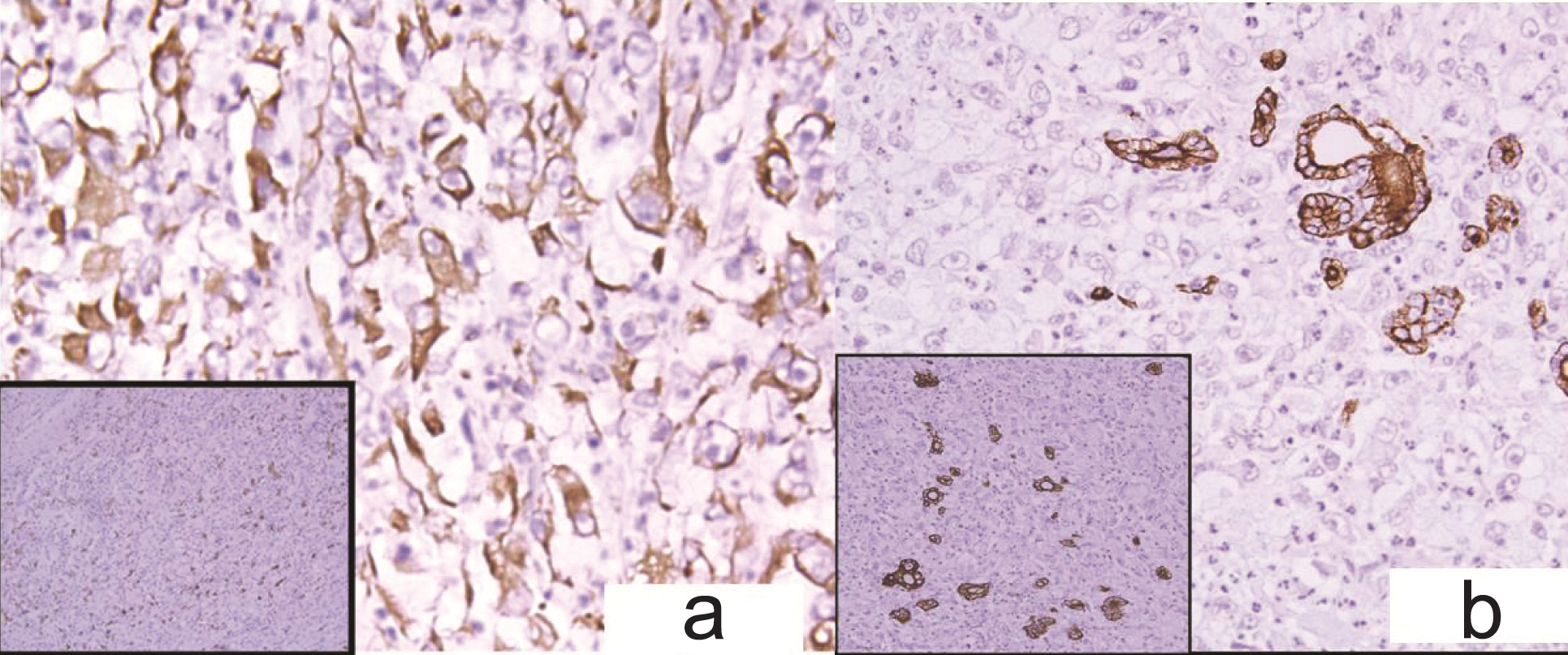
A 52-year-old woman, presented with rapidly increasing neck mass, pain and constriction. No symptoms of hypo or hyperthyroidism were present. On examination there was a large lobulated neck mass measuring 8x7 cm. Ultrasonography showed an 8.5x6.9 cm hypoechoic thyroid mass with dense central calcification. On Computed Tomography (CT) scan a tumor mass involving whole of thyroid measuring 8.5x7.9x2.3cm that invaded the strap muscles, trachea and carotid sheath was found. Fine needle aspiration of neck mass yielded predominantly haemorrhagic aspirate. Giemsa stained smears showed few atypical spindled out cells with hyperchromatic nucleus, 1-2 prominent nucleoli and focal areas of necrosis in a predominantly haemorrhagic background ([Table/Fig-3b], Inset) Normal thyroid follicles were not seen. Immunocytochemistry was not performed due to lack of cellularity. On the basis of few atypical cells, focal areas of necrosis and radiological findings a possibility of malignant lesion was suggested. She underwent total thyroidectomy. No normal thyroid was seen on gross examination and the tumor showed large areas of necrosis [Table/Fig-3a]. Microscopically the tumor showed a storiform pattern at places and was composed of pleomorphic epithelioid to spindle shaped cells with frequent mitosis and no lymphovascular space invasion [Table/Fig-3b,c]. Immunohistochemistry in both the cases showed tumor cells immunoreactive for Vimentin (Vim) ([Table/Fig-3d] Inset, [Table/Fig-4a]) and CD-68 ([Table/Fig-3d], [Table/Fig-4a] inset); negative for thyroglobulin, desmin, pan Cytokeratin (CK), calcitonin, CD34, and S100. Cytokeratin [Table/Fig-4b] and Thyroglobulin ([Table/Fig-4b], inset) highlighted the preserved thyroid follicles in between the tumor cells. Thus a diagnosis of (MFH-T) was suggested. Both the patients were thoroughly investigated clinicoradiologically for a possible primary elsewhere but no focus was identified.
(a) Case 2-Gross: Thyroid gland is replaced by tumor with areas of necrosis. (b) Tumor cells arranged in storiform pattern (Arrow) (H&E, x400). Inset: FNAC smears show scattered atypical spindle cells with focal areas of necrosis (Giemsa, x40). (c): Tumor cells in sheets with areas of necrosis. (Arrow) (H&E, x200). (d) Tumor cells express CD 68 (Immunohistochemistry with DAB as chromogen x400); Inset: Vimentin positive tumor cells (x100).
(a) Case 2-Diffuse Vimentin positivity. (Immunohistochemistry with DAB as chromogen x400) Inset: CD-68 positive tumor cells. (Immunohistochemistry with DAB as chromogen x100) (b) Cytokeratin positive remnant thyroid follicles. (Immunohistochemistry with DAB as chromogen x400) Inset: Thryroglobulin positive remnant thyroid follicles (Immunohistochemistry with DAB as chromogen x200).
Thus, a final diagnosis of primary thyroid MFH-T was made in both these cases. Both the patients were treated surgically with complete thyroidectomy along with surrounding soft tissue and lymph node resection. The patients could not be given any adjuvant therapy as the post operative course was not good. Both the patients unfortunately died of severe respiratory distress following pulmonary metastasis and thromboembolism.
Case 3
A 65-year-old woman presented with midline neck swelling since six months with hoarseness of voice since six days. On examination thyroid swelling was 15x8 cm in size. CT scan showed 8.3x10.5x 7.6 cm mass lesion with areas of calcification and necrosis suggestive of malignant growth thyroid gland (possibly papillary carcinoma) [Table/Fig-5a]. Mass was displacing the larynx and trachea to the left side. Fine needle aspiration cytology (FNAC) was reported as a malignancy- possibly papillary carcinoma thyroid. Positron emission tomography (PET) scan showed heterogeneous uptake in right lobe and isthmus of thyroid gland with a nodule in pulmonary parenchyma suggestive of metastases [Table/Fig-5b].
(a) Case 3- CT scan: heterogeneous enhancing mass in thyroid gland with solid areas and necrosis in the centre. (b) Positron emission tomography scan- heterogeneous uptake in thyroid gland right lobe and isthmus. (c) Gross-tumor is replacing whole of thyroid gland. Cut section: Grey white to yellow, firm lobulated mass.
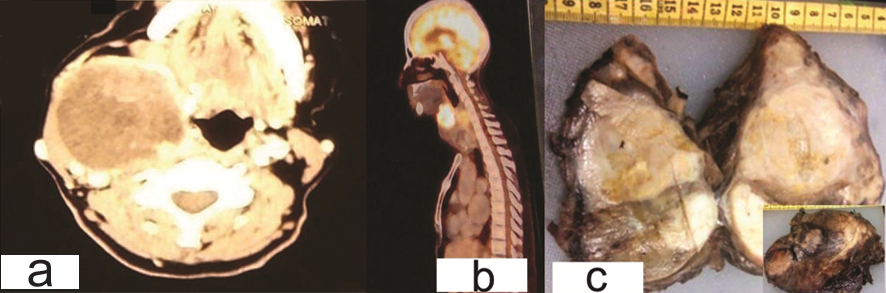
Total thyroidectomy with lymphadenectomy was performed. Grossly tumor was replacing whole of thyroid gland. On cut section a grey white to yellow, firm lobulated mass with focal areas of whorling and necrosis was observed. ([Table/Fig-5c] and Inset) Periphery of the growth showed compressed normal thyroid parenchyma.
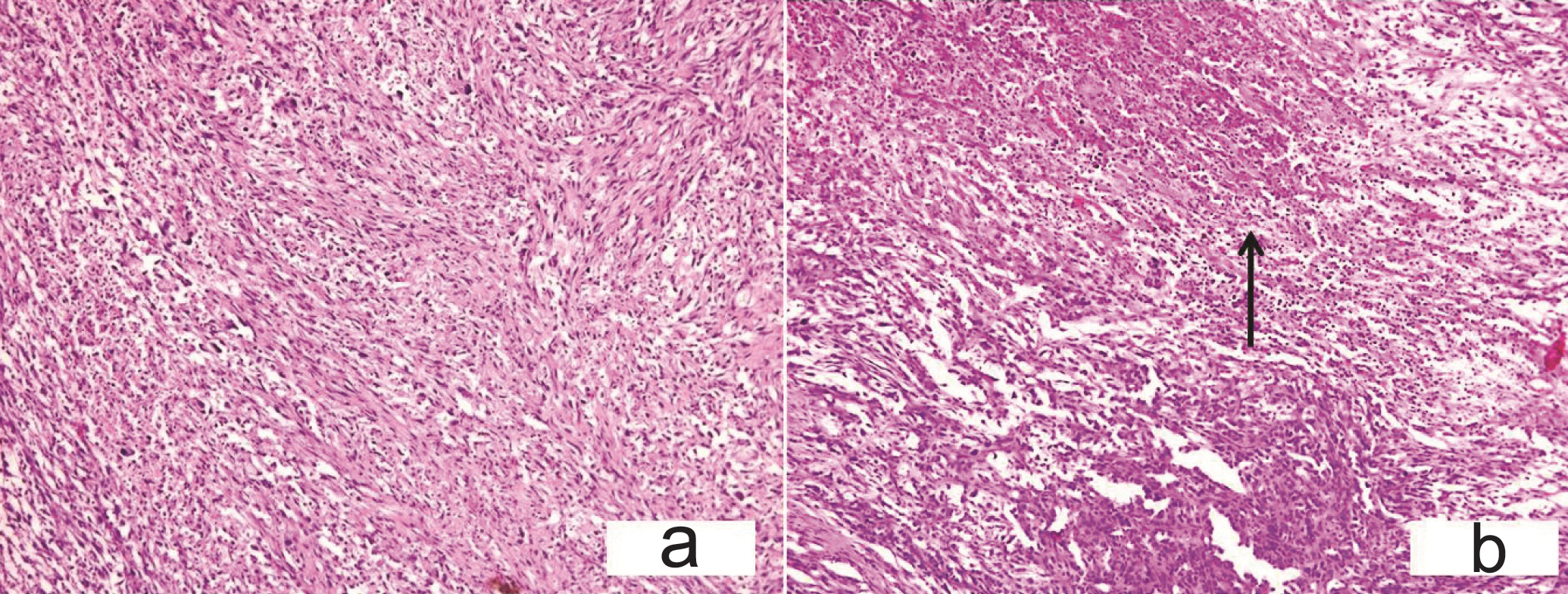
Histopathological examination revealed a tumor composed of spindled cells having cigar shaped nucleus, moderate degree of pleomorphism. Large areas of necrosis along with neutrophilic infiltrate and multinucleated giant cells were noted [Table/Fig-6a,b,7a]. Tumor was infiltrating the surrounding strap muscle. Lymph node metastasis was present. The residual interspersed thyroid follicles were present in between the tumor tissue [Table/Fig-7b] and the surrounding thyroid showed features of multinodular goitre. Mitotic count was 3-4/high power field. The differential diagnoses considered were: (AC-T), medullary carcinoma thyroid (spindle cell variant) and sarcoma thyroid.
(a) Case 3-Spindle cells in sheets and whorls replacing whole of thyroid gland. (H&E, x200): 6b) Large areas of necrosis (arrow) with neutrophilic infiltrate (H&E x200).
(a) Case 3-Cigar shaped nucleus and mild pleomorphism with thyroid follicles at periphery (H&E x200); (b) Residual normal thyroid follicles in between tumor cells (H&E x200); (c) Tumor cells are immunoreactive for Smooth muscle actin. (Immunohistochemistry with DAB as chromogen x200); (d) -Tumor cells do not express thyroglobulin; residual follicles immunoreactive for Throglobulin (Immunohistochemistry with DAB as chromogen x200).
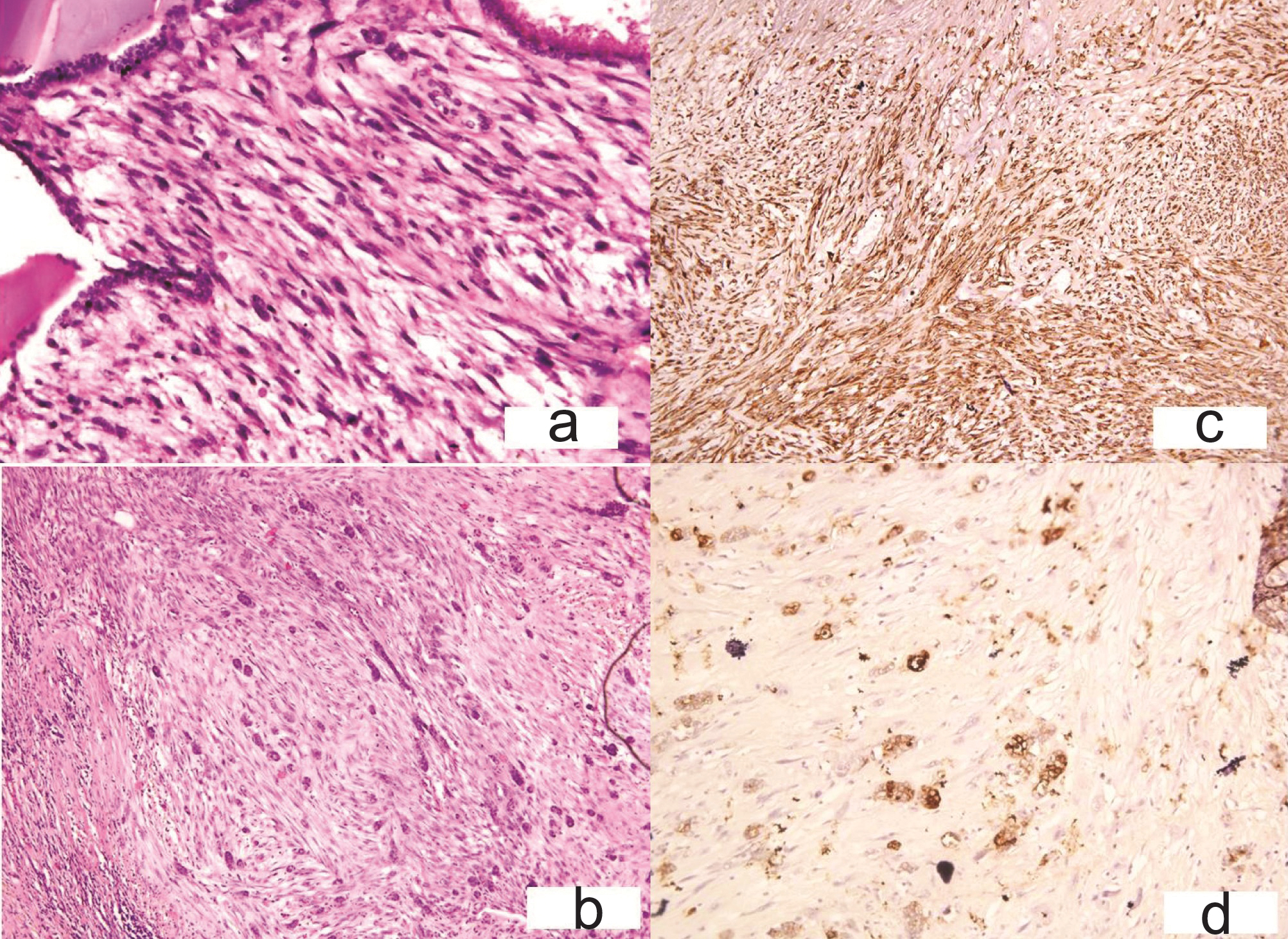
The cells were immunoreactive for Vimentin, smooth muscle actin [Table/Fig-7c] and stained negatively for calcitonin, thyroglobulin [Table/Fig-7d], CK and CD 68. Secondary leiomyosarcoma (LHS-T) was ruled out after the gynaecological, systemic examination and PET scan. On basis of histopathological examination, immunohistochemistry and clinicoradiological work up patient was diagnosed as primary leiomyosarcoma thyroid (LMS-T). No adjuvant therapy could be given as the patient’s clinical condition didn’t allow it. Summary of the three cases is provided in [Table/Fig-8].
Summary of the three cases.
| AGE (Years) | SEX | Surrounding Thyroid | IHC | Diagnosis |
|---|
| CASE 1 | 63 | F | Grave’s | CD 68 + Vimentin + | MFH |
| CASE 2 | 52 | F | Normal | CD 68+ Vimentin + | MFH |
| CASE 3 | 65 | F | multinodular goitre | SMA+ Vimentin+ | LMS |
LMS: Leiomyosarcoma, MFH: Malignant Fibrous Histicytoma, IHC: Immunohistochemistry SMA = Smooth muscle actin
Discussion
Sarcomas involving the thyroid gland are extremely rare. One of the first reported case of primary thyroid (MFH-T) was by Zahradka et al., in 1989 with less than 30 cases in English literature so far [1–3] including a series of 12 cases by Zeng et al., [4].
Leiomyosarcoma of the thyroid gland (LMS-T) is rare with less than 25 cases reported in literature [5,6] and comprises 0.014% cases of primary sarcomas of thyroid gland [5]. MFH-T and LMS-T present at usually advanced stage with distant metastasis and carries a poor prognosis [1–7]. The present communication adds three new cases to the literature on sarcomas of thyroid gland with an emphasis of differential diagnosis of spindle cell lesions of thyroid.
MFH-T commonly occurs in the extremities and retroperitoneum; followed by head and neck region and rarely as primary in thyroid gland [2,3,7]. MFH affecting the thyroid gland could be primary or metastatic, secondary being rarer [2–4,6]. The site of origin is uncertain, although MFH has been thought to arise from the fascia surrounding the thyroid gland [2]. The mean age of presentation is 57 years [1–4]. It occurs more commonly in women than men, with a reported female to male ratio of 3:1 [1–4,7]. The tumor usually presents as a hard, fixed, rapidly enlarging neck mass or neck pain and cough within months of initial presentation [3,4]. Classically it is composed of plump spindle cells arranged in storiform pattern with high pleomorphism and tumor giant cells [1–4,7].
LMS-T may originate from smooth muscles of the capsular vessels [5]. LMS-T shows fascicles of elongated tumor cells with spindled out cytoplasm and elongated nuclei with blunt ends [5]. Secondary sarcomas involving the thyroid gland must be ruled out after extensive investigation before giving a diagnosis of primary thyroid sarcomas [4,5].
All the cases in this present case series match the clinical profile by presence in elderly ladies and manifesting as a rapidly increasing thyroid mass with pain. All the three cases had pulmonary metastases at the time of diagnoses and had a poor clinical outcome.
The nearest histological differential of MFH-T and LMS-T is AC-T which can mimic it both clinically and radiologically. Immunohistochemistry plays a crucial role in the diagnosis. Variants of AC-T with sarcomatous elements and with rhabdomyosarcomatous differentiation can be totally indistinguishable (positive for CK; vimentin, desmin and actin may be present in sarcomatous component). CD-68 expressed in AC-T tumor giant cells is known to be negative in tumor cells) thus distinguishing with MFH and always negative for SMA and H- caldesmon thus differentiating from LMS [3]. Spindle cell variant of medullary carcinoma is immunoreactive for synaptophysin, cytokeratin, calcitonin and negative for Smooth Muslce Active (SMA), desmin and thyroglobulin. MFH is positive for vimentin, CD68, α1-antitrypsin, α1-antichymotrypsin, and vimentin, but negative for actin, CK and desmin [3]. LMS is immunoreactive for vimentin, smooth muscle actin, H- caldesmon and desmin [5]. Primary sarcomas of thyroid also need to be ruled out including fibrosarcoma (Vimentin positive, negative for SMA, CD 68, CK) and synovial sarcoma (expresses CK and Vimentin). Epithelial tumors with spindling like squamous cell carcinoma (Vimentin, SMA negative) and SETTLE (spindle epithelial tumor with thymus-like differentiation seen in younger age group and comprises both of epithelial and spindle out cells which are cytokeratin positive; and does not show atypia) can be differentiated on histological analysis. Another close differential is metastasis of sarcoma to the thyroid gland which needs to be ruled out clinically and radiologically [2–5]. ([Table/Fig-9] describes the close differential diagnosis of sarcomas of thyroid gland)
Differential diagnosis of sarcomas of thyroid gland.
| LMS | MFH | AC-T | MCT (Spindle cell variant) | METASTASICMFH /LMS |
|---|
| Incidence | 0.014% | RARE | ~5% | RARE | RARE |
| Origin | Smooth muscles of capsular blood vessels | Uncertain originProbably fascia surrounding thyroid | Undifferentiated | Para follicularC-cells | ? multipotent fibroblast |
| Age/sex | >55, F>M | >65, F>M | >60, F>M | 40-60, F>M | >50, F>M |
| Morphology | Pleomorphic spindle cells in sheets & whorls, necrosis & mitosis | Plump spindle cells in storiform pattern,Tumor giant cells | Varied | Spindle out cells | Varied |
| IHC | SMA+, Vimentin + | CD 68+, Vimentin+ | CK+TG – SMA-, CD 68- | Calcitonin +TG- | Vimentin+CD68/ SMA+TG- |
| Prognosis | Poor | Poor | Dismal | Better | Poor |
LMS: Leiomyosarcoma, MFH: Malignant Fibrous Histiocytoma, AC-T: Anaplastic Carcinoma Thyroid (TG- Thyroglobulin, TTF- Thyroid transcription factor, Ck- cytokeratin, SMA = Smooth muscle actin, IHC: Immunohistochemistry.
The diagnosis of MFH-T and LMS-T carries grave prognostic implications with surgery being the mainstay of treatment. In study by Zeng et al., in MFH patients receiving radiotherapy died within 16 months [4]. Radiotherapy was of some benefit in single MFH case [8].
Raspollini et al., observed increased expression of c-kit in leiomyosarcomas [9]. However, the effect of imatinib to improve the survival rate needs to be studied as no significant effect of imatinib was noted on survival by Day AS et al., [10]. Estrogen and progesterone receptors are sporadically expressed in LMS-T suggesting hormonal therapy may benefit these patients [5].
Conclusion
Primary MFH-T and LMS-T are rare neoplasms that need to be differentiated from AC-T, metastatic sarcomas, spindle cell variant of medullary carcinoma, synovial sarcoma, fibrosarcoma; final diagnosis rests on histopathology and immunohistochemistry. Surgery is the mainstay of treatment while effect of combination with radiotherapy and chemotherapy needs to be observed in larger number of patients for improving patient survival.
LMS: Leiomyosarcoma, MFH: Malignant Fibrous Histiocytoma, AC-T: Anaplastic Carcinoma Thyroid (TG- Thyroglobulin, TTF- Thyroid transcription factor, Ck- cytokeratin, SMA = Smooth muscle actin, IHC: Immunohistochemistry.
[1]. Zahradka W, Trietz M, Lhotzky R, Malignant fibrous histiocytoma of the thyroidZ Gesamte Inn Med 1989 44:563-65. [Google Scholar]
[2]. Malandrinou F, Tseleni-Balafouta S, Kakaviatos N, Singhellakis P, Primary malignant fibrous histiocytoma on the thyroidHormones (Athens) 2002 1:255-59. [Google Scholar]
[3]. Hsu KF, Lin YS, Hsieh CB, Yu JC, Duh QY, Sheu LF, Primary malignant fibrous histiocytoma of the thyroid: review of the literature with two new casesThyroid 2008 18:51-55. [Google Scholar]
[4]. Zeng Q, Tang PZ, Xu ZG, Qi YF, Wu XX, Liu WS, Primary malignant fibrous histiocytoma of the thyroidEur J Surg Oncol 2009 35:649-53. [Google Scholar]
[5]. Conzo G, Troncone G, Docimo G, Santini L, Cytologically undetermined follicular lesions: surgical procedures and histological outcome in 472 casesAnn Ital Chir 2012 84:251-56. [Google Scholar]
[6]. Tanboon J, Keskool P, Leiomyosarcoma: a rare tumor of the thyroidEndocr Pathol 2013 24(3):136-43. [Google Scholar]
[7]. Ackerman L, Romyn A, Khedkar N, Kaplan E, Malignant fibrous histiocytoma metastatic to the thyroid glandClin Nucl Med 1987 12:648-49. [Google Scholar]
[8]. Yavuz AA, Cobanoglu U, Genc M, Yavuz MN, Kosucu P, Aslan MK, Malignant fibrous histiocytoma of the thyroid gland: recurrence treated by radiotherapyJ Otolaryngol 2005 34:216-20. [Google Scholar]
[9]. Raspollini MR, Aminni G, Villanucci A, Pinzani P, Simi L, Paglierani M, Taddei GL, C-kit overexpression in patients with uterine leiomyosarcomas: a potential alternative therapeutic treatmentClin Cancer Res 2004 10:3500-03. [Google Scholar]
[10]. Day AS, Lou PJ, Lin WC, Chou CC, Over-expression of c-kit in a primary leiomyosarcoma of the thyroid glandEur Arch Otorhinolaryngol 2007 264:705-08. [Google Scholar]