The purpose of this case report is to describe the usefulness of Periosteal Pedicle Graft (PPG) as a barrier membrane and Demineralized Freeze-Dried Bone Allograft (DFDBA) for bone regeneration in periradicular bone defect. A patient with intraoral discharging sinus due to carious exposed pulp involvement was treated by PPG and DFDBA. Clinical and radiological evaluations were done immediately prior to surgery, three months, six months and one year after surgery. Patient was treated using split-thickness flap, PPG, apicoectomy, defect fill with DFDBA and lateral displacement along with suturing of the PPG prior to suturing the flap, in order to close the communication between the oral and the periapical surroundings through sinus tract opening. After one year, successful healing of periradicular bone defect was achieved. Thus, PPG as a barrier membrane and DFDBA have been shown to have the potential to stimulate bone formation when used in periradicular bone defect.
Apicoectomy, Bone healing, Defect fill, Intraoral sinus, Vascularized periosteum
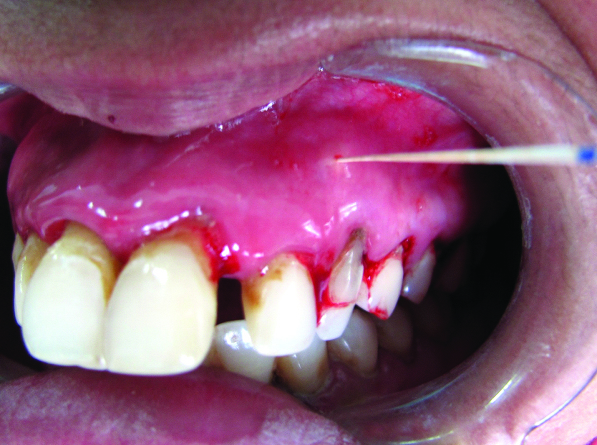
A 23-year-old female patient reported with the chief complaint of pus discharge from upper front tooth region. On intraoral examination, a draining sinus tract at labial surface of the upper left canine (tooth no #23) was present due to carious exposed pulp involvement [Table/Fig-1]. Probing the area yielded a 3-4 mm pocket depth at different points on the buccal surface, giving a definite clinical impression of no apicomarginal involvement and presence of generalized Chronic Periodontitis (ChP). Radiographical examination revealed a periapical radiolucency at the apex of tooth no #23 [Table/Fig-2a].
A sinus tract opening in tooth no #23.
a)Preoperative radiograph of periapical region; b) Radiograph after endodontic treatment.
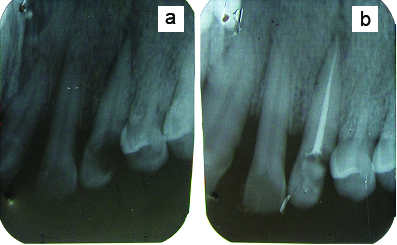
The patient underwent basic periodontal treatment of phase I therapy including scaling, root planing and instructions for proper oral hygiene measures. Endodontic treatment including root canal treatment and restoration was done prior to surgery [Table/Fig-2b]. After this, generalized ChP was treated by thorough root planing, subgingival curettage, irrigation with antimicrobial agent and systemic antibiotics.
This case report was approved by the institutional ethical committee for human subjects and also adhered to the Helsinki declaration of 1975, as revised in 2000. At the time of surgery, patient was instructed to do presurgical rinse by 0.2% chlorhexidine solution. The facial skin around the mouth was cleaned with spirit and scrubbed by 7.5% povidone iodine solution. Intraoral surgical site was painted with 5% povidone iodine solution [1].
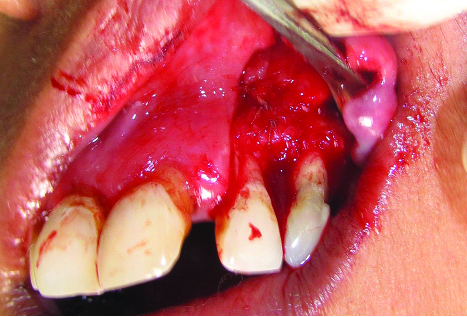
After proper part preparation, 2% lignocaine hydrochloride with 1:80,000 adrenaline was administered to anaesthetize left infraorbital and naso-palatine nerves. After local anaesthesia, split-thickness flap that consisted of buccal intrasulcular incision including one tooth mesial and two teeth distal to the lesion, and two vertical incisions were elevated to fully uncover the buccal surface of the root and access the donor periosteum site [Table/Fig-3a]. All incisions were made in a supra-periosteal fashion. A #15 Bard-Parker blade was used to incise the gingiva so as to provide an approximately 1.5–2 mm uniformly thick flap wall. The flap was extended under tension as the incision proceeded apically. Care was exercised not to tear or perforate the flap base accidentally with the scalpel. After elevation of the flap, the granulomatous tissue over the involved tooth was removed from the bone defect margin by sharp dissection. PPG consisting of connective tissue with the periosteum was obtained from the distal area adjacent to the defect for use as a biological barrier membrane. Four incisions were made, two vertical incisions distal to the defect that connected with a third submarginal horizontal incision and a small releasing incision in the base of pedicle, connective tissue with periosteum as PPG was harvested with a periosteal elevator [Table/Fig-3b]. PPG was intact at base and stored under the split-thickness flap until needed. Debridement of the bony defect was done [Table/Fig-4a]. Root planing of the exposed root with a gracey curette was done. Apical root-end resection [Table/Fig-4b] was done with round diamond bur at high speed, with sterile water coolant, removing approximately 3 mm of the root apex. A 3-mm deep root-end cavity was prepared and filled with light cure glass ionomer cement. Irrigation was done with 100 mg/ml of doxycycline solution for five minutes in order to remove the smear layer, to expose the collagen matrix and to prevent the degradation of collagen [Table/Fig-5].
a) Split-thickness flap extended till second premolar; b) Periosteal pedicle graft reflected from premolar region.
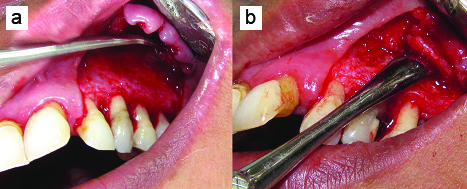
a) After debridement of bone defect; b) After 3-mm of apical root-end resection.
Irrigation of periradicular bone defect done with doxycycline solution.
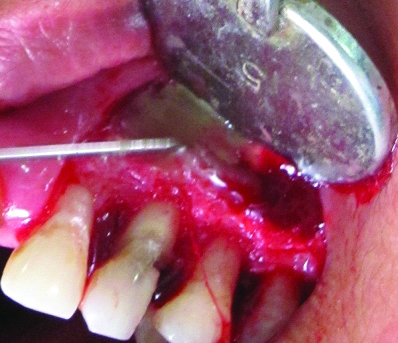
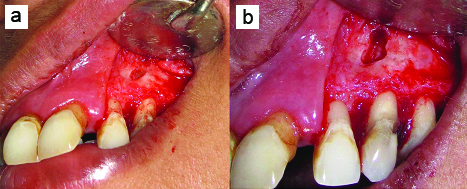
Periradicular bone defect was filled with DFDBA (Tata Memorial Hospital, Tissue Bank, Mumbai, India) [Table/Fig-6] and PPG was sutured over the filled defect to cover it [Table/Fig-7]. Split-thickness flap was sutured at its original position with 3-0 black silk suture [Table/Fig-8]. Finger pressure was then applied for five minutes on the operated area. No periodontal dressing was used to allow good chemical plaque control by chlorhexidine. Immediate postoperative intraoral periapical radiograph was taken [Table/Fig-9a].
Periradicular bone defect filled with DFDBA.
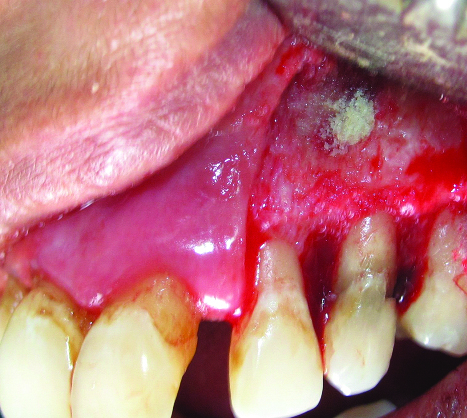
Periosteal pedicle graft sutured over the filled defect to cover it.
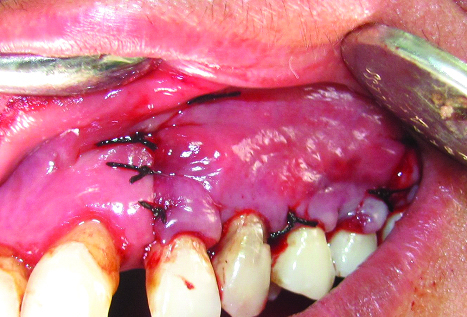
Split-thickness flap sutured at its original position to close the wound.
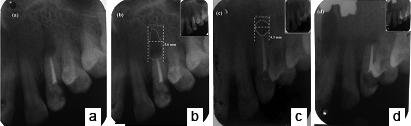
a) Immediate postoperative radiographical appearance; b) Three months postoperative IOPA radiograph showed reduced radiolucency; c) Six months postoperative IOPA radiograph showed incomplete bone healing; d) One year postoperative IOPA radiograph showed complete bone healing.
Antibiotic (amoxicillin 500 mg, 1 tablet every 8 hours, for 7 days) and analgesic (nimesulide 100 mg, 1 tablet every 12 hours, for 3 days) were prescribed. Patient was instructed to be extremely cautious during mastication of meals and no toothbrushing or chewing on the operated area for three weeks. After this period patient was advised to mechanical cleaning of the operated area using an extra soft toothbrush by coronally directed “roll” technique. Plaque control was obtained by 0.2% chlorhexidine rinse, twice daily during the first two weeks, and then application of 0.2% chlorhexidine gel onto the operated area for another two weeks.
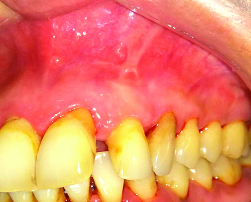
Sutures were removed two weeks after surgery. Clinical and radiological follow-up was performed at three months, six months and one year after surgery. At three and six months postoperative, periapical radiolucency was substantially reduced but still not healed completely [Table/Fig-9b, c]. After one year recall examination, radiographical evaluation showed complete bone healing [Table/Fig-9d] and tooth was asymptomatic, with healthy periodontal tissues and normal probing depths [Table/Fig-10].
One year postoperative showed healthy periodontal tissues.
Discussion
In this case, periapical bone defect with erosion of buccal cortical plate and concomitant marginal lesion without communication were present, hence, it belongs to Class II a according to Von Arx and Cochran classification of periradicular lesions, which is based on location, extension or pathway of infection [2].
After periradicular surgery, processes of regeneration are affected by destruction of the cortical plate and the presence of dentoalveolar sinus tract [3]. Presence of a dentoalveolar sinus tract may allow epithelial migration and proliferation of gingival connective tissue from the adjacent oral mucosa into periradicular bone defect and prevent healing and the formation of normal trabecular bone [4]. In addition, the epithelialized tract may permit further contamination through ingress of bacteria and bacterial byproducts from the oral cavity [5]. As the periosteum is damaged in such cases, this enhances the chance of unreliable repair [6]. Generally, the prognosis of periradicular surgery varies between 25-90% [7]. This wide variation is a reflection of the multiplicity of factors that affect the outcome, including the size of the lesion, periodontal involvement, perforation of the cortical plate, and persistent sinus tracts. When bony destruction of the pathological process includes the cortical plate, the prognosis for success is reported to be 37% [5].
When a sinus tract is present, the periosteum is often damaged by the infective process [6]. Periosteum is important for the healing of a periapical bone defect following periradicular surgery because it may act both as a source of osteo-competent cells and as a barrier membrane against the infiltration of epithelial cells, bacteria and bacterial byproducts into the healing site [8]. Periosteum is composed of three zones. Zone-1 is cambium layer (closest to the bone) contains osteoblasts, osteoblast progenitor cells, and multipotent stem cells. Zone-2 is matrix layer contains fibroblasts, fibroblast progenitor cells, and dense vascular plexus. Because of this layer, periosteum is highly vascular. Zone-3 is collagenous layer (outermost layer) contains dense collagen fibers. Zone-2 and Zone-3 are together known as fibrous layer. Thus, periosteum consists of two main layers, an inner cambium layer and an outer fibrous layer. The inner cambium layer has osteogenic capacity, as it contains multipotent stem cells and progenitor cells thus, it can be used as an autogenous barrier membrane which can be harvested in sufficient quantities from adjacent to surgical site and is easy harvest; it has high vasculo-proliferative and neuro-trophic activities as well. Moreover it releases Vascular Endothelial Growth Factor (VEGF) which helps in angiogenesis and wound healing, secretes periostin that favors osteoblast attachment and spreading. All the above mentioned properties make periosteum suitable as a graft [9]. PPG are used for many purposes as for treatment of recession, furcation and intrabony defect. Periosteal graft as barriers in periradicular surgery of two cases was reported by Tobon-Arroyave SI et al. They found complete bone healing in one case while the region of periapical radiolucency substantially reduced but still not healed completely in another case [3]. Lokade J et al., reported two cases of apicoectomy followed by bone defect filled with PerioGlas and covered with a resorbable Guidor membrane. After 24 months of follow-up, they found no clinical signs or symptoms associated with the lesions and radiographic examination showed progressive resolution of radiolucency [10]. Marin-Botero ML et al., compared healing responses to periosteal sliding grafts and polyglactin 910 periodontal mesh used as GTR materials/techniques when both periapical and periradicular bone loss are present. They found similar enhancements of the clinical outcome of periradicular surgery in terms of periapical healing, gain of periodontal support, pocket depth reduction and minimal recession of the gingival margin [11].
DFDBA is the most widely used allograft material in periodontics, in part due to its availability, safety, osteoinductive and osteoconductive properties. Osteoinductive property is due to presence of Bone Morphogenic Proteins (BMPs), which stimulate local cell cycles to produce new bone and osteoconductive property is due to freezedrying process, which destroys cells while maintaining cellular morphology and chemical integrity. These two properties of DFDBA enhance periodontal regeneration and/or bone fill. Human histologic studies have shown that DFDBA can promote the formation of a new attachment apparatus on previously diseased root surfaces-including new cementum, bone and periodontal ligament [12].
The clinical and radiographical evidence of newly formed bone does not necessarily indicate regeneration; hence, histological evaluation is ideally needed to confirm the efficacy of periosteal grafts in promoting true bone regeneration. It has been stated that lifting of the periosteum or surgical trauma results in a marked generative activity by cells and osteogenesis [13]. However, in the wound, the cells of the cambium layer of the periosteum are destroyed by the reflective forces used to elevate the flap [14]. The periosteum does not function in bone repair until the osseous excisional wound is almost filled with woven bone trabeculae of endosteal tissue origin and this occurs at 14 days post-surgery and suggests that there is an inductive influence from the new bone to the reforming periosteum to develop osteogenic potential and become a functioning periosteum [15].
In terms of viability, vascularized periosteum is superior to free periosteum. The osteogenic capacity of vascularized periosteum is less affected by the environment of the recipient site as compared with free periosteum. Special attention was paid to raise vascularized periosteum and the results reveal a good osteogenic capacity in the short-term, possibly stimulated by the surgical trauma.
The advantages of PPG are that periosteum can be easily harvested, relatively abundant and healing of donor and recipient areas was well tolerated by patient. Another advantage is that the configuration of the periosteum can be adjusted to the shape of the recipient site. The only disadvantages are profuse bleeding during elevation of split-thickness flap and the moderate degree of difficulty encountered during separation of periosteum, which is firmly attached to the underlying bone. This last problem can be overcome with practice and expertness of operator.
Conclusion
The results of the present case report suggest that the use of PPG as a barrier membrane and DFDBA for the treatment of periradicular bone defect contribute to a successful clinical outcome.
[1]. Singh AK, Kiran P, The periosteum eversion technique for coverage of denuded root surfaceJ Indian Soc Periodontol 2015 19:458-61. [Google Scholar]
[2]. Von Arx T, Cochran DL, Rationale for the application of the GTR principle using a barrier membrane in endodontic surgery: A proposal classification and literature reviewInt J Periodontics Restorative Dent 2001 21:127-39. [Google Scholar]
[3]. Tobón-Arroyave SI, Domínguez-Mejía JS, Flórez-Moreno GA, Periosteal grafts as barriers in periradicular surgery: Report of two casesInt Endod J 2004 37:632-42. [Google Scholar]
[4]. Dahlin C, Lindhe A, Gottlow J, Nyman S, Healing of bone defects by guided tissue regenerationPlast Reconstr Surg 1988 81:672-76. [Google Scholar]
[5]. Skoglund A, Persson G, A follow-up study of apicoectomized teeth with total loss of the buccal bone plateOral Surg Oral Med Oral Pathol 1985 59:78-81. [Google Scholar]
[6]. Pecora G, De Leonardis D, Ibrahim N, Bovi M, Cornelini R, The use of calcium sulphate in the surgical treatment of a ‘through and through’ periradicular lesionInt Endod J 2001 34:189-97. [Google Scholar]
[7]. Gutmann JL, Harrison JW, Surgical Endodontics 1991 1st editionBoston, MA, USABlackwell Scientific Publications [Google Scholar]
[8]. Enriquez FJJ, Vieyra JP, Ocampo FP, Relationship between clinical and histopathologic findings of 40 periapical lesionsDentistry 2015 5:277-83. [Google Scholar]
[9]. Singh N, Uppoor A, Naik DG, Bone’s smart envelope-The periosteum: Unleashing its regenerative potential for periodontal reconstructionInternational Journal of Contemporary Dental and Medical Reviews 2015 :2015 [Google Scholar]
[10]. Lokade J, Wankhade S, Chandak M, Lanjewar A, Guided tissue regeneration principle with inserts of PerioGlas in endodontics surgery: Two case reportsInt J Prosthodont Rest or Dent 2013 3:72-77. [Google Scholar]
[11]. Marin-Botero ML, Dominguez-Mejia JS, Arismendi-Echavarria JA, Mesa-Jaramillo AL, Florez-Moreno GA, Tobon-Arroyave SI, Healing response of apicomarginal defects to two guided tissue regeneration techniques in periradicular surgery: A double-blind, randomized-clinical trialInt Endod J 2006 39:368-77. [Google Scholar]
[12]. Bowers G, Chadroff B, Carnevale R, Mellonig J, Corio E, Emerson J, Histologic evaluation of new attachment apparatus formation in humans: Part IIIJ Periodontol 1989 60:683-93. [Google Scholar]
[13]. Goldman HM, Smukler H, Controlled surgical stimulation of periosteumJ Periodontol 1978 49:518-22. [Google Scholar]
[14]. Harrison JW, Jurosky KA, Wound healing in the tissues of the periodontium following periradicular surgery. II. The dissectional woundJ Endod 1991 17:544-52. [Google Scholar]
[15]. Harrison JW, Jurosky KA, Wound healing in the tissues of the periodontium following periradicular surgery. III. The osseous excisional woundJ Endod 1992 18:76-81. [Google Scholar]