Biofilm Formation by Drug Resistant Enterococci Isolates Obtained from Chronic Periodontitis Patients
Sonia Bhonchal Bhardwaj1, Manjula Mehta2, Shaveta Sood3, Jyoti Sharma4
1 Assistant Professor, Department of Microbiology, Dr. Harvansh Singh Judge Institute of Dental Sciences and Hospital, Chandigarh, India.
2 Associate Professor, Department of Microbiology, Dr. Harvansh Singh Judge Institute of Dental Sciences and Hospital, Chandigarh, India.
3 Assistant Professor, Department of Periodontics, Dr. Harvansh Singh Judge Institute of Dental Sciences and Hospital, Chandigarh, India.
4 Assistant Professor, Department of Microbiology, Dr. Harvansh Singh Judge Institute of Dental Sciences and Hospital, Chandigarh, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Sonia Bhonchal Bhardwaj, Assistant Professor, Department of Microbiology, Dr Harvansh Singh Judge Institute of Dental Sciences and Hospital, Chandigarh-160014, India.
E-mail: sbbhardwaj2002@yahoo.com.
Introduction
Enterococci are an important cause of opportunistic nosocomial infections and several multidrug resistant strains have emerged. The severity of periodontal diseases is managed by reduction in the pathogenic bacteria. There is a need to assess the prevalence and antibiotic susceptibility of enterococci colonizing the periodontal pocket and correlate its biofilm formation ability because oral biofilms provide a protective environment and are a reservoir of bacterial colonization of the gingival crevice.
Aim
To investigate possible association between antibiotic susceptibility and biofilm formation in enterococci isolates from chronic periodontitis patients.
Materials and Methods
This retrospective study was conducted at Dr. Harvansh Singh Judge Institute of Dental Sciences and Hospital, Punjab University, Chandigarh from January 2015 to October 2015. Sterile paper points were inserted in the periodontal pocket of 100 subjects and put in a transport media. Forty -six isolates were identified as enterococci. The isolates were further examined for their ability to form biofilm by microtitre plate assay and antimicrobial susceptibility testing was done by disc diffusion method for clinically relevant antibiotics.
Results
Significant relationship (p<0.001) was found between biofilm production with antibiotic resistance to Vancomycin, Erythromycin, Ciprofloxacin, Tiecoplanin, Amoxycillin and Gentamycin.
Conclusion
The study demonstrates a high propensity among the isolates of Enterococci to form biofilm and a significant association of biofilm with multiple drug resistance.
Antibiotic resistance, Biofilm matrix, Enterococcus, Periodontal disease
Introduction
Enterococci are Gram positive bacteria inhabiting the gastro intestinal tract. Now, they are an important cause of nosocomial infections but were initially regarded as non virulent [1]. Enterococci are now ranked as the third most common nosocomial bacterial pathogen after coagulase negative Staphylococci, Staphylococcus aureus [2]. The emergence of multidrug resistant strains of enterococci to commonly used antimicrobials like tetracycline and erythromycin is a matter of concern [3].
The two most common enterococci species are Enterococcus faecalis and Enterococcus faecium. Both the species can produce biofilm, which is a population of cells surrounded by a matrix of macromolecules like polysaccharides, proteins, lipids and extracellular DNA [4]. E. faecalis has been recovered from periodontal pockets in 1% to 51.8% of periodontitis patients [5,6]. It has been seen that the presence of E. faecalis in the pockets of chronic periodontitis was significantly higher than that of a treated group [5]. The subgingival E. faecalis has been found resistant to routine antimicrobial agents in a high proportion [7,8]. Furthermore, recently it has been shown that enterococci are adept at acquiring transferable antimicrobial resistance and are likely to be a reservoir for diverse mobile genetic elements [9]. There is a need to assess the antibiotic susceptibility of enterococci colonizing periodontal pocket and also assess the biofilm formation ability as it may enhance the enterococcal pathogenesis in infections. The role of enterococcal biofilm and antibiotic resistance in chronic periodontitis remains unclear.
The objective of the present study was to determine the biofilm formation ability of enterococci strains in periodontitis and their antimicrobial susceptibility.
Materials and Methods
A total of 100 subjects (52 males and 48 females) attending the periodontitis clinic of Dr Harvansh Singh Judge Institute of Dental Sciences and Hospital, Punjab University, Chandigarh were examined for presence of periodontitis. Patients with chronic periodontitis were included in the test group. The control group consisted of thirty healthy persons who did not have obvious dental disease. The study was approved by the ethics committee of Punjab University. Patients who were pregnant, allergic, having diabetes mellitus, on antibiotic therapy or undergoing orthodontic therapy were excluded. The study was approved by the ethics committee of Punjab University. Patients were informed of the study protocol and aim and written consent was obtained. For control, ATCC 14506 Enterococcus faecalis strain was used.
Sampling Procedure: The sample sites was isolated. After isolation with cotton rolls and removal of saliva and supragingival deposits, one to two sterile, absorbent paper points were introduced into each periodontal pocket for 30-60 seconds. After removal, all paper points per patient were pooled and transferred immediately to test tubes containing glucose azide broth (HiMedia Laboratories, Mumbai) and taken to the laboratory within four hours for microbiological analysis.
Bacterial Isolation and Identification: The samples were inoculated onto the blood agar (HiMedia Laboratories, Mumbai) plates and incubated in microaerobic condition. Every growth showing Gram positive cocci, positive bile esculin, positive 6.5% Nacl tests, Catalase negative was processed for further biochemical identification [10].
Antimicrobial Susceptibility Testing
The antibiotic susceptibility of the test strains to different antibiotics (amoxicillin, ciprofloxacin, erythromycin, vancomycin, gentamycin, tiecoplanin) purchased from HiMedia Laboratories, Mumbai was determined by standard disc diffusion method (Kirby Bauer sensitivity test) [11] and interpreted according to the Clinical and Laboratory Standard Institute (CLSI) guidelines [12]. The test was performed on Mueller Hinton agar (HiMedia) and results read after 24 hours of incubation at 370C.
Biofilm Assay
Biofilm formation was performed by microtitre plate assay [13]. Inoculums were prepared by growing in Brain Heart Infusion broth (BHI) containing 0.25% glucose and incubated at 370C overnight. Overnight broth cultures were diluted 1:20 in fresh BHI broth supplemented with glucose. 200 ul of diluted strain was dispensed into triplicate wells in a single column of a sterile 96 well flat bottom plate (APW) and incubated at 370C for 24 hours. The microtitre plate was gently tapped to remove the planktonic cells and wells were washed three times with 300 ul of sterile Phosphate Buffer Saline (PBS). The plates were inverted and allowed to dry for one hour at room temperature. Biofilms were then fixed with 200 ul of 0.5% aqueous crystal violet solution for 15 minutes and the wells were subsequently washed thrice with sterile PBS to remove the excess crystal violet. Microtitre plates were then inverted on a filter paper and air dried. 200 ul of 80:20 (v/v) mixtures of ethyl alcohol and acetone was added to solubilized bound crystal violet. Absorbance of the extracted crystal violet was measured at 550 nm Automatic Microplate Reader (APW). For positive control, Staphylococcus epidermidis and for negative control, non biofilm forming bacteria Salmonella typhi was used in each plate. All biofilm assays were repeated three times. The cut-off value (ODc) was established. ODc, defined as three Standard Deviations (SD) above the mean OD of the negative control. The final OD value was taken as mean OD value of test strain divided by ODc value of the triplicate assays. Any OD value above the cut-off value was indicative of biofilm production.
Statistical Analysis
SPSS software version 16.0 (IBM, SPSS statistics) was used for statistical analysis. t-test and Chi–square test was performed for data analysis. p-values below 0.001 were considered to be significant.
Results
Forty six enterococci isolates were obtained from subgingival samples collected from 70 periodontitis patients. No enterococci isolate was obtained from control group. The mean age of all the participants in the study was 40.77 years with male participants being 52 and female participants being 48 in number. All the patients had mild to moderate periodontitis. Subjects were in the age range of 18-75 years. Based on the biochemical reactions, the species of E. faecalis were 39(84.78%) followed by E. faecium 7 (15.21%). The distribution of 46 enterococci isolates among patients in association of sex, smoking and oral hygiene is shown [Table/Fig-1].
Distribution of enterococci isolates in association of sex, smoking and oral hygiene among periodontitis patients.
| Characteristics | Enterococci isolates (n=46) (%) | p value |
|---|
| MaleFemale | 24/46 (52.2%)22/46 (47.8%) | NS |
| SmokerNon Smoker | 40/46 (87.0%)6/46 (13.0%) | 0.001 |
| Poor oral hygieneGood oral hygeine | 35/46 (76.1%)11/46 (23.9%) | 0.001 |
NS-non significant
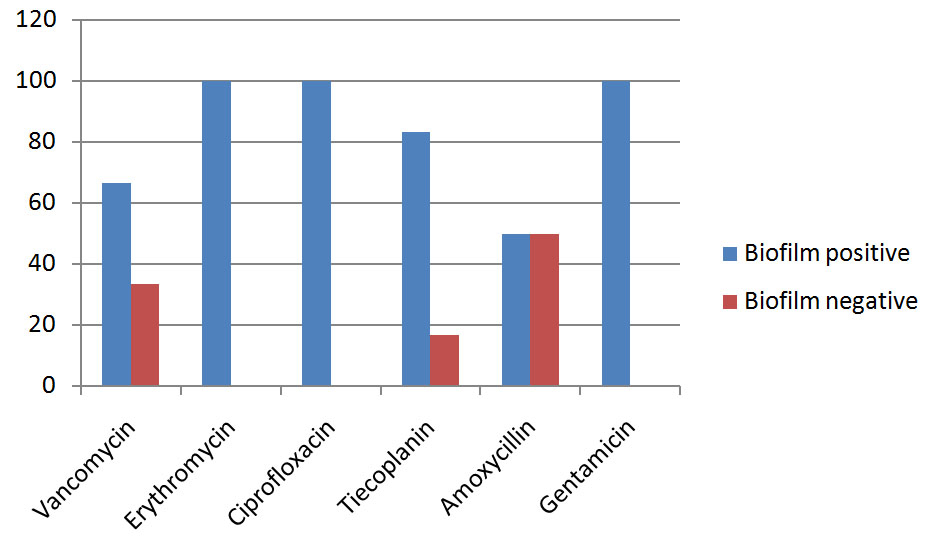
Among the enterococci isolates tested for antimicrobial susceptibility resistance to erythromycin were 3/46(6.5%), ciprofloxacin 4/46 (8.7%), tiecoplanin 6/46 (13.0%), amoxycillin 2/46 (4.3%), gentamycin 4/46 (8.7%), vancomycin 6/46(13.3%) [Table/Fig-2].
Association between biofilm production and antibiotic susceptibility pattern of enterococci isolates.
| Antimicrobials | No of resistantIsolates producingBiofilm/Total NoOf resistant isolates. | No of sensitive isolates producing biofilm/Total No of sensitive isolates. | p value |
|---|
| Vancomycin | 4/6 (66.7%) | 33/40(82.5 %) | <0.001 |
| Erythromycin | 3/3(100.0%) | 35/43(81.4%) | <0.001 |
| Ciprofloxacin | 4/4(100.0%) | 34/42(81.0%) | <0.001 |
| Tiecoplanin | 5/6(83.3%) | 33/40(82.5%) | <0.001 |
| Amoxycillin | 1/2(50.0%) | 37/44(84.1%) | <0.001 |
| Gentamycin | 4/4(100.0%) | 33/42(78.57%) | <0.001 |
Quantitative microtitre assay for biofilm formation was positive in 39/46 (84.78%) isolates. The remaining isolates were non biofilm producers considered as negative. E. faecalis strains positive for biofilm production was 71.8% and E. faecium was 25.6%. Statistical analysis showed significant relationship of biofilm formation with antibiotic resistance. Biofilm formation was significant in resistant isolates (p<0.001) [Table/Fig-2]. Comparison of biofilm positive and biofilm negative isolates among antibiotic resistant enterococci has been shown in [Table/Fig-3].
Comparison of biofilm positive and biofilm negative isolates among antibiotic resistant enterococci. The y axis represents the percentage of the isolates resistant to different antibiotics and the X axis represents resistance to the antibiotic mentioned.
Discussion
Enterococci are able to colonize the oral cavity particularly in patients with periodontitis or root canal infections associated with oral mucosal lesions and in immunocompromised patients [14]. It has been implicated that enterococci might influence periodontal antimicrobial therapy and contribute to disease progression in severe subgingival infections [15]. In this study, majority of the isolates identified were E. faecalis (84.78%) followed by E. faecium (15.21%). The study reveals that smoking and poor oral hygiene are important predisposing factors for infection with enterococci. These results agree with another study that show patients with periodontitis had more diverse combination of species as compared to healthy persons [16] and that smoking has been shown to influence oral microbiome composition [17].
Importance of biofilm formation has been described in the control of microbial infection in several areas because the biofilm can increase resistance to various physical and chemical agents especially antibiotics [18]. Biofilm formation is indirect evidence of adhesiveness and microtitre plate assay is the indirect way to measure adhesion of enterococci. In this study, enterococci isolates were resistant to multiple antibiotics. Survival advantages conferred by the biofilm community include resistance to phagocytosis and to antimicrobial agents [19].
Significant relationship was found between biofilm production with antibiotic resistance to Vancomycin, Erythromycin, Ciprofloxacin, Tiecoplanin, Amoxycillin, Gentamycin. Biofilm formation was significantly more in Erythromycin resistant enterococci isolates (100%) vs sensitive isolates (81.4%), Ciprofloxacin resistant (100.0%) vs sensitive (81.0%), Tiecoplanin resistant isolates (83%) vs sensitive (82%) and Gentamycin resistant (100.0%) vs sensitive (80.5%) and also in the Vancomycin resistant and Amoxycillin resistant the biofilm formation was significantly more vs the sensitive isolates. The study showed that almost all enterococci strains exhibited biofilm forming ability in vitro. Biofilm exhibits more resistance to broad spectrum antibiotics [19]. This supports that biofilm adds to the virulence profile of microorganisms [20]. Other studies particularly in urinary tract infection patients have also shown a possible relationship between virulence profile and biofilm formation by enterococci [21,22]. A variety of mechanisms for the increased antimicrobial resistance of microorganism in a biofilm have been proposed including extracellular matrix in biofilm might physically restrict the diffusion of antimicrobial agents, nutrient and oxygen depletion within the biofilm cause some bacteria to enter a stationary state, in which they are less susceptible to microbial killing, a subpopulation of bacteria might differentiate into a phenotypically resistant state and some organisms in biofilm have shown to express biofilm specific antimicrobial resistance genes [23]. Recently, it has been shown that extracellular DNA (eDNA) in the biofilm matrix protects microbial cells from a variety of antimicrobial agents [4].
The present study points to the importance of biofilm susceptibility testing in clinical settings because the difference in susceptibility is remarkable under various growth conditions. The variations observed in these clinical isolates suggests that biofilm formation may be a factor when considering the virulence phenotype of periodontal strains in general. However, biofilm formation is an indirect evidence of adhesiveness and microtitre plate assay is the indirect way to measure adhesion in vitro.
Limitation
A limitation of this study is the lack of use of animal models as representative models of periodontitis in humans as the experimental models have the ability to mimick the pathogenesis of natural disease [24].
This study also highlights the role of enterococci in implication of periodontal disease. In a recent study of very small sample size, E.faecalis has been reported as a pathogen which is a critical marker of disease stage of chronic periodontitis patients [25]. Studies also suggest that periodontal infections and oral bacteria may be a risk factor for a number of prevalent systemic diseases [26]. Therefore, close attention should be given to periodontitis patients who may harbor pathogenic bacteria in the oral cavity in order to reduce the risk for development of systemic infections.
Conclusion
Dental Plaque is regarded as a major causative factor in dental diseases like dental caries and periodontal disease. Most of the antimicrobial resistant strains were biofilm producers as seen in our study. Hence, biofilm susceptibility testing which reflects more natural conditions in plaque related dental diseases, can lead to more appropriate evidence based therapeutic strategies.
NS-non significant
[1]. Marra A, Dib-Hajj F, Lamb L, Kaezmarek F, Shang W, Beckius G, Enterococcal virulence determinants may be involved in resistance to clinical therapyDiag Microbiol Infect Dis 2007 58:59-65. [Google Scholar]
[2]. Zoletti GO, Pereira EM, Schuenk RP, Teixeira LM, SiqueiraJr JF, Santos KR, Characterization of virulence factors and clonal diversity of Enterococcus faecalis isolates from treated dental root canalsRes Microbiol 2011 162:151-58. [Google Scholar]
[3]. Souto R, Colombo APV, Prevalence of Enterococcus faecalis in subgingival biofilm and saliva of subjects with chronic periodontal infectionArch Oral Biol 2008 53:155-60. [Google Scholar]
[4]. Jakubovics NS, Grant Burgess J, Extracellular DNA in oral microbial biofilmsMicrobes and Infection 2015 17(7):531-37. [Google Scholar]
[5]. Balaei-Gajan E, Shirmohammadi A, Abashov R, Agazadeh M, Faramarzei M, Detection of Enterococcus faecalis in subgingival biofilm of patients with chronic refractory PeriodontitisMed Oral Pathol Oral Cir Buccal 2010 15:e667-70. [Google Scholar]
[6]. Sun J, Song X, Assessment of antimicrobial susceptibility of Enterococcus faecalis isolated from chronic periodontitis biofilm vs planktonic phaseJ Periodontol 2011 82:84 [Google Scholar]
[7]. Sun J, Song X, Kristiansen BE, Kjaereng A, Willems RJ, Eriksen HM, Occurrence, population structure and antimicrobial resistance of Enterococci in marginal and apical periodontitisJ Clin Microbiol 2009 47:2218-25. [Google Scholar]
[8]. Al-BadahSuliman AH, Ibrahim SS, Abdel Nasser, Salamah AA, Ibrahim Shebl, Salah S, Clonal diversity and antimicrobial resistance of Enterococcus faecalis isolated from endodontic infectionsElectronic journal of Biotechnology 2015 18:175-80. [Google Scholar]
[9]. Song X, Sun J, Milkalsen T, Roberts AP, Sundsgford A, Characterisation of the plasmidome within Enterococcus faecalis isolated from marginal periodontitis patients in NorwayPLOS One 2013 8:4 [Google Scholar]
[10]. Teixeira LM, Facklam RR, EnterococcusIn Manual of Clinical Microbiology, 8th edn ed. Murray PR, Baron EJ, Jorgensen JH, Pfaller MA and Yolken RH 2003 WashingtonASM Press:422-433. [Google Scholar]
[11]. Bauer AW, Kirby WM, Sherris JL, Turck M, Antibiotic susceptibility testing by a standardized single disk methodAm J Clin Pathol 1966 45(4):493-96. [Google Scholar]
[12]. Clinical and Laboratory standard Institute. Performance standard for antimicrobial susceptibility testing: Twentieth International Supplement M100 S20 CLSI, Wayne PA, USA, 2010 [Google Scholar]
[13]. Stepanovic S, Vukovic D, Hola V, Bonaventura GD, Djukic S, Irkovic IC, Quantification of biofilm in microtitre plates: overview of testing conditions and practical recommendations for assessment of biofilm production by StaphylococciAPMIS 2007 115:891-99. [Google Scholar]
[14]. Pinheiro ET, Anderson MJ, Gomes BPFA, Ducker DB, Phenotypic and genotypic identification of Enterococci isolated from canals of root filled teeth with periapical lesionsOral Microbiol Immunol 2007 21:137-44. [Google Scholar]
[15]. Lee HJ, Kim JK, Cho JY, Lee JM, Hong SH, Quantification of subgingival bacterial pathogens at different stages of periodontal diseasesCurrMicrobiol 2012 65:22-27. [Google Scholar]
[16]. Abusleme L, Dupuy AK, Dutzman N, Silva N, Burleson JA, Strausbaugh LD, The subgingival microbiome in health ad periodontitis and its relationship with community biomass and inflammationThe ISME Journal 2013 7:1016-25. [Google Scholar]
[17]. Shchipkova AY, Nagaraja HN, Kumar PS, Subgingival microbial profiles of smokers with periodontitisJ Dent Res 2010 89:1247-53. [Google Scholar]
[18]. De la Fuente-Nunez C, Reffuveille F, Fernandez L, Hancock RE, Bacterial biofilm development as a multicellular adaptation: antibiotic resistance and new therapeutic strategiesCurr Opin Microbiol 2013 16:580-89. [Google Scholar]
[19]. Mathur T, Singhal S, Khan S, Upadhyay DJ, Fatma T, Rattan A, Detection of biofilm formation among clinical isolates of Staphylococci: An evaluation of three different screening methodsIndian J Med Microbiol 2006 24:25-29. [Google Scholar]
[20]. Toledo-Aruna A, Vall J, Solano C, Arrizubeita MJ, Cucarella C, Lamata M, The Enterococcal surface protein, ESP, is involved in E. faecalis biofilm formationAppl and Environmental Microbiol 2001 67:4538 [Google Scholar]
[21]. Mohamed JA, Singh K, Huang W. Influence of clinical origin and by Enterococcus faecalis. In program Abstracts of the 43 rd Annual Interscience conference on antimicrobial agents and chemotherapy. Abstract B821. American Soceity for Microbiology. Washington. DC.2003; Pp. 42 [Google Scholar]
[22]. Kafil HS, Mobarez, AM, Assessment of biofilm formation by Enterococci isolates from urinary tract infections with different virulence profilesJournal of King Soudi University-Science 2015 27:312-17. [Google Scholar]
[23]. Patel R, Biofilms and antimicrobial resistanceClin Orthop Relat Res 2005 437:41-47. [Google Scholar]
[24]. De Molon RS, De Avila ED, Nogueira AVB, De Souza JAC, Campos MJA, De Andrade CR, Evaluation of the host response in various models of induced periodontal disease in miceJ Periodontol 2014 85:465-77. [Google Scholar]
[25]. Yoon DL, Shukho K, Song H, Kim YG, Lee J M, Kim J, Detection of bacterial species in chronic periodontitis tissues at different stages of disease severityJ BacteriolVirol 2015 45(4):364-71. [Google Scholar]
[26]. Li X, Koltveit KM, Transtad L, Olsen I, Systemic diseases caused by oral infectionClin Microbiol Rev 2000 3:547-51. [Google Scholar]