The reproductive cycle in rodents called the estrous cycle is short and precise. It undergoes four phases namely proestrus, estrus, metestrus and diestrus and lasts for 4 to 5 days. Estimation of the estrous cycle is crucial to assess the functioning status of the female reproductive system in mice when used as an experimental model in reproductive biology. Ever since, the methods evolved to estimate the estrous cycle from vaginal smear in guinea pig by Stockard and Papanicolaou [1] and visual method by Allens [2] in his classical research on the estrous cycle in mice, there has not been a single gold standard method for its precise estimation. Vaginal smear technique in guinea pig by Stockard and Papanicolaou has been the accepted standard method for other mammalian species. The vaginal smear technique is not without disadvantages; however, it ensures accurate estimation of the estrous stage. The reproductive period of mice begins with the opening of vagina at around 26th day of birth. Vaginal opening is an apoptosis mediated event [3] and is an important secondary sexual character in mice. It is used as an external indicator for the puberty onset which can be identified by simple visual inspection.
The proestrus stage corresponds to the human follicular phase in which there is increase in 17-β Estradiol levels and small surge in Prolactin. In response to the elevated Estradiol level, Luteinizing Hormone (LH) and Follicle Stimulating Hormones (FSH) are released from anterior pituitary into the circulation. The peak in FSH levels Signals ovulation and entry into the estrus stage. In this stage, there is sharp decline of 17-β Estradiol levels and Prolactin levels peak. It is the period of heat or sexual receptivity. Metestrus and Diestrus are homologous to the human early and late secretory phases respectively in which progesterone levels peak [4].
The physiological changes occurring in the estrous cycle in mice can be assessed by different methods like vaginal cytology [2,5,6], electrical impedance method [7], biochemical analysis of urine [8] and visual examination of vagina [2,9]. All the four stages of estrous cycle can be estimated using vaginal smear method, but it is labour intensive and time consuming. Champlin et al., described the estimation of estrous cycle in mice by the gross appearance of vagina. This method is relatively quicker, easier than vaginal cytology method and accurate [9]. In the present study, an attempt had been made to compare the routinely employed vaginal lavage and visual method in the estimation of estrous cycle in mice.
Materials and Methods
A cross-sectional study was done on 60 healthy female swiss albino mice from the central animal facility of Sri Ramachandra University with the approval of Institutional Animal Ethics Committee. (Approval No: IAEC/XLVII/SRU/476/2016). The experiment was conducted between April and May 2016 in compliance with the ethical guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India, New Delhi. All the animals were housed in polypropylene cages and maintained in 12 hour light dark cycle with relative humidity of 25±2°C and were fed normal pellet diet with free access to water ad libitum.
Assessment of Vaginal Opening in Mice
Vaginal opening occurs due to increase in the estradiol levels in mice. Unlike rats, where vaginal opening occurs during first ovulation, in mice vaginal opening occurs 10 days prior to vaginal cornification and the onset of estrous cycle [5]. In the present study the mice were monitored for the vaginal opening from 24 to 30 days after birth by vaginal inspection. The vaginal opening occurred normally within this duration in the mice in the present study. After the vaginal opening, the mice were kept in adaptation period for a week followed by which estrous cycle was estimated by both visual and vaginal lavage method.
Visual Method
In this method, vagina of each animal was examined carefully to avoid misinterpretation due to cursory observation. The investigation room was provided with adequate illumination because light source is extremely important for visual examination. LED light was avoided as it has a purple hue which will make the detection difficult as observed by Shannon Byers et al., [10]. Each mouse was held in the non dominant hand and laid in the restraint with the forepaws. The tail of the mouse was lifted gently and the vulva was examined by the method described by Champlin et al., [9]. Photographs were taken in Sony cyber shot digital camera (16.2 megapixels) for the purpose of documentation.
Vaginal Lavage Method
In this method, the vaginal cells were flushed by introducing small amount of distilled water or saline through pipette and placing few drops of cell suspension in a glass slide for microscopic examination. The vaginal secretion is normally composed of three types of cells namely leucocytes, cornified epithelial cells and nucleated epithelial cells. The estrous cycle is estimated by the proportion of these cells in this method. In the present study, vaginal smears were collected by non invasive method proposed by Ashleigh C. Mclean [4].
For cytological assessment, 0.1% crystal violet stain was used. It was prepared by adding 0.1g of crystal violet powder in 100 ml of double distilled water (ddH20). Autoclaved ddH20 was used in the present study for sterile vaginal lavage.
Procedure
A 100μl of ddH20 was taken in a sterile latex bulb. The mouse was kept in a restrainer with its forepaws. The tail was elevated to visualise the vagina. The end of the latex bulb containing 100μl of ddH20 was placed at the entrance of the vaginal canal. Care was taken to avoid penetration into the vaginal orifice. The bulb was gently pressed and ddH20 was expelled into the vaginal canal. The pressure on the bulb was slowly released and the water was drawn back into the tip. The step was repeated 4-5 times in the same bulb. The fluid was then placed in a glass slide, air dried and stained with 0.1% crystal violet stain. The slide was overlaid with a cover slip and examined under light microscope.
Results
Visual Method
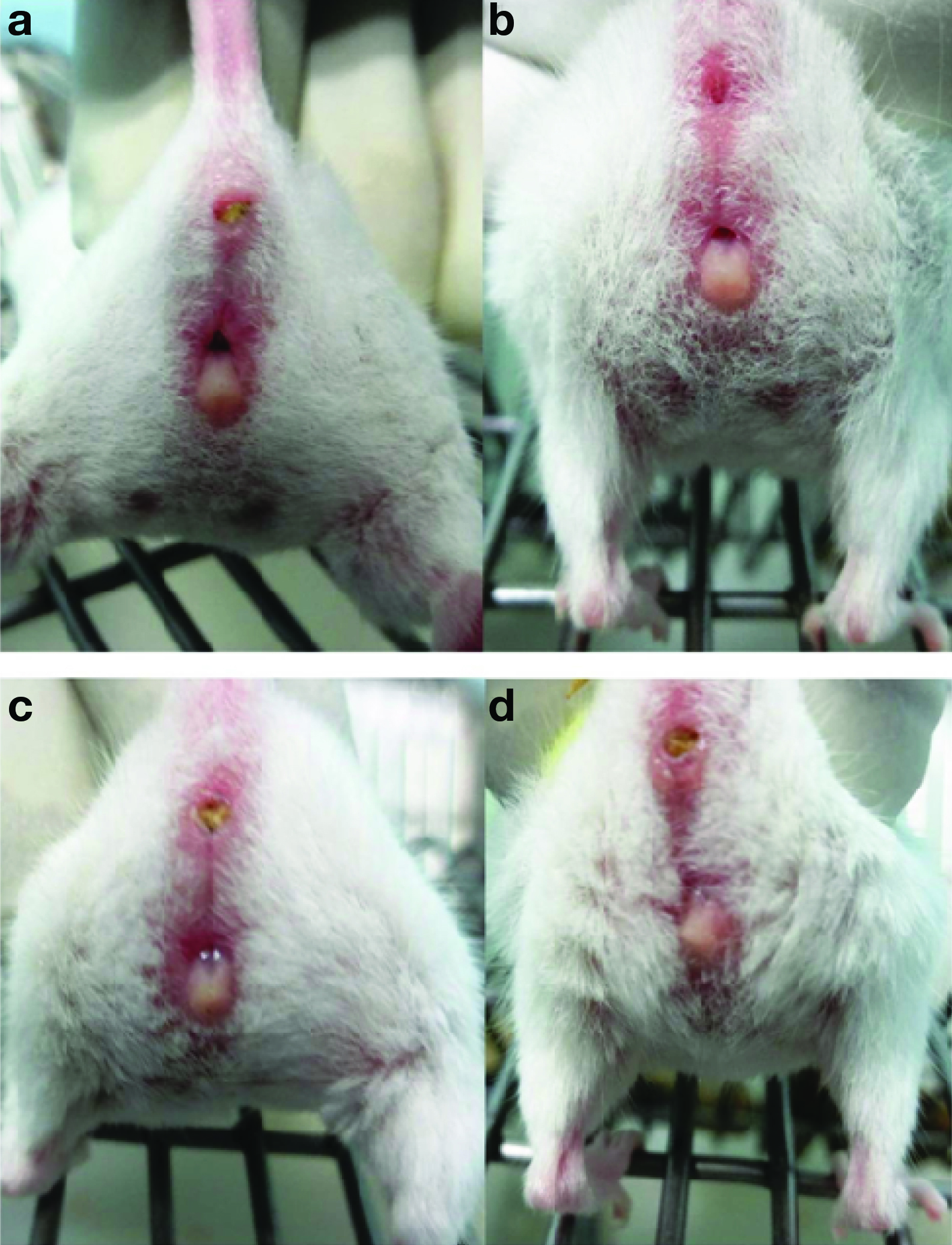
In proestrus, the vaginal opening was wide, moist and the tissues appeared pink. Striations were seen in both the dorsal and the ventral lips of the vulva. In estrus phase, the vagina appeared similar to proestrus, but it was less pink, less moist but striations were more pronounced at this stage. Metestrus phase was characterised by pale, dry vaginal opening which was sloughed with white cellular debris. In diestrus, vaginal opening was very moist, too small and closed in some mice with no tissue swelling [Table/Fig-1].
Appearance of vagina in different phases of estrous cycle.
a-Proestrus, b-Estrus, c- Metestrus, d- Diestrus
Vaginal Lavage Method
Proestrus
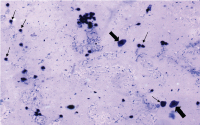
It is the short phase of estrous cycle and refers to the pre ovulatory day. In the present study, it was characterised by the presence of nucleated epithelial cells which were seen in clusters or individually. A few anucleated cells and cornified cells were also present [Table/Fig-2].
Proestrus – large nucleated epithelial cells are present (large arrow). Few anucleated cells and cornified cells were also seen (Small arrow). Original objective magnification 10X;
Estrus
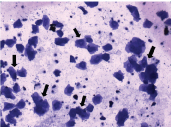
It was characterised by the presence of cornified epithelial cells which were abundant and often non nucleated. The cytoplasm is granular and shape of the cells is irregular [Table/Fig-3].
Estrus stage – presence of large number of cornified epithelial cells (large arrows). Original objective magnification 10X.
Metestrus
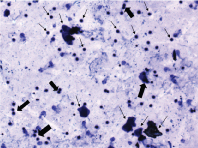
It is a brief stage which consisted of large number of leucocytes and small number of large, non granular and non nucleated cornified epithelial cells. The leucocytes formed small, tightly packed clumps of cells [Table/Fig-4].
Metestrus stage – Presence of large number of leucocytes and small number of large, non granular and non nucleated cornified epithelial cells (small arrows). Leucocytes are seen in clumps (large arrows). Original objective magnification 10X;
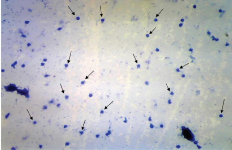
Diestrus
Diestrus was identified with the large number of leucocytes. Some degree of epithelial and cornified cells was also present. The cells at this stage did not form tightly packed clumps [Table/Fig-5].
Diestrus stage – Presence of large number of leucocytes (small arrows). Original objective magnification 10X.
In the present study, most of the animals exhibited regular cycles. Cycle length of less than 4 days and more than 6 or 7days were considered irregular [11]. Five animals in the present study showed, continuous estrus throughout the smearing period. Acyclic smear is reported in two animals in the present study in which there were repeated diestrus smears for 12 to 16days which is due to the increased sensitivity of the animals that led to pseudo pregnancy. The samples of acyclic animals showed more amount of mucus. Such changes were monitored by visual examination method also.
Discussion
The endocrine milieu of the experimental animal can be ascertained by the estimation of estrous cycle especially for mating studies. The proportion of cells present in smears is indicative of the estrous cycle stage of the animal in which the chance of the number of cells showing characteristic of two stages is high. Such transition stage samples are common if smears are taken very early or late in the day. Likewise, short phases like proestrus can be missed if samples are taken early morning because in some females proestrus does not appear until mid-morning. So, it would be advisable to take samples between 10.00 and 13.00 [11]. The occurrence of extended estrous cycle increases with age or due to increased period of light illumination in the animal facility. Acyclic smears are largely due to the stimulation of cervix during smearing and it occurs more commonly in vaginal swab and vaginal pipette methods. However, in some hypersensitive animals non invasive methods can also lead to acyclicity as in the present study.
Emmens CW., recommended gentle scraping of vaginal wall for better vaginal smears [12]. Epithelial cells can be obtained from the vaginal walls in scraping method rather than the sloughed cells in lavage method. But, vaginal scraping method may not be applicable for long term study of estrous cycle in smaller species like mice because of its potential stress. The smears obtained by lavage method are clearly indicative of the estrous stage and so undue stress can be avoided. The vaginal smears can be examined unstained in microscopes in studies where the general cyclic pattern is to be established. But when estrous cycle is to be evaluated as an endpoint measure, vaginal smears need to be fixed with 95% ethanol, dried and stained [13].
The housing of animals and atmospheric control in animal facility has an impact in the cyclic pattern in mice. Female mice housed with male mice in the same room showed more regular cycles than in all female environment where prolonged anestrus periods were observed [14]. But in the present study, most of the animals exhibited regular cycles in an all female environment. Estrous cycle can also be affected by continuous illumination in which case there will be persistent estrus [15]. A complete estrous cycle can be defined by the period from one proestrus to another. Some authors conclude metestrus as a transition period during the early part of diestrus (diestrus 1) and its smear consists of leucocytes, cornified and round epithelial cells [13].
In the present study, the animals were not restrained for a longer period in vaginal lavage method to avoid stress which may affect the estrous cycle. The animals initially were restless but after the adaptation period the animals co-operated for the procedure. The estimation of the estrous cycle was done at the same period of the day at 10.00 am and vaginal lavage yielded higher cellularity samples for microscopic examination in the present study.
In visual method of examination, initial assessments may be difficult and take time. According to Shannon L. Byers et al., if a person is trained well, approximately 100 female mice can be evaluated in 10 to 15minutes, no equipment is required. In visual detection, proestrus and estrus can be identified clearly, but other stages will be difficult to identify with this method alone [10]. In the present study, the evaluation of estrous cycle by visual method coincides with the evaluation by vaginal lavage method. The main disadvantage in visual method is the bias and cursory observation of the examiner. The risk of pseudo pregnancy and mechanical trauma in vaginal swab method makes it inapplicable in long term estrous cycle estimation. Hence, for accurate estimation of estrous cycle non invasive method as an identification tool is recommended.
Limitation
The present study is done in a specific period of time in a limited number of mice. Though, the findings are conclusive of the precise estimation of the estrous cycle, a larger number of animals for a considerably longer duration of experiment is necessary. The present study is done in mice, however the non-invasive method can be replicated in other species like rats, guinea pigs and rabbits so that a standard protocol for the estimation of estrous cycle in experimental animals can be determined.
Conclusion
The visual method provides a rapid means of assessing the estrous cycle in mice through practice and careful observations. But with visual method alone, it is difficult to assess the transitional stages of estrous cycle. The non invasive method of estrous cycle estimation is ideal for the precise estimation of the estrous cycle and this method can be applied for a long term as it is non invasive and there is no risk of pseudo pregnancy as in vaginal smear method.