Primary Cutaneous Histoplasmosis Masquerading as Lepromatous Leprosy
Jenna Blah Bhattacharya1, Poonam Rani2, Radhika Aggarwal3, Seema Kaushal4
1 Senior Resident, Department of Pathology, Maulana Azad Medical College, New Delhi, India.
2 Senior Resident, Department of Pathology, Maulana Azad Medical College, New Delhi, India.
3 Junior Resident, Department of Pathology, Maulana Azad Medical College, New Delhi, India.
4 Associate Professor, Department of Pathology, Maulana Azad Medical College, New Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Jenna Blah Bhattacharya, House No. 2102, Tower-C, Amrapalli Eden Park, Block-F, Sector-50, Noida-201301, Uttar Pradesh, India.
E-mail: jennamamc@gmail.com
Histoplasmosis is a genus of dimorphic fungi having various varieties of which the commonest one causing infection is Histoplasma capsulatum known to cause histoplasmosis. It has a varied disease spectrum ranging from an acute infection to chronic disease especially in lungs, disseminated disease and cutaneous disorder. Histoplasma capsulatum usually causes subclinical infection and serious infections only manifest in immunocompromised patients. Frank cases of infection are seen in pulmonary histoplasmosis. The spores of these organisms are seen to be strongly associated with droppings of birds and bats. A combination of these droppings and some soil types provide for an excellent environment for the proliferation of spores. Pulmonary histoplasmosis and disseminated disease are very common in AIDS patients and are a great cause of morbidity and mortality in these patients. Primary cutaneous histoplasmosis is very rare and occurs due to penetrating injuries. Once diagnosis is made, the lesions respond very well to oral itraconazole, fluconazole or amphotericicn B. We report a rare case of Cutaneous Histoplasmosis (CHP) in a 70-year-old male with complaints of multiple nodules all over his body in a HIV seronegative and otherwise immunocompetent patient.
Nodulo-ulcerative lesions, Lepromatous leprosy, Mycotic infection
Case Report
A 70-year-old male presented to the dermatology outpatient department with history of multiple erythematous to skin coloured nodules all over the face, head and neck region and upper trunk for one and a half years. Examination revealed multiple painless nodules of varying sizes predominantly over the face and neck [Table/Fig-1], some of which showed hyperpigmentation. No crusting or discharge was noted.
Clinical photograph showing papulonodular skin lesions all over the face.
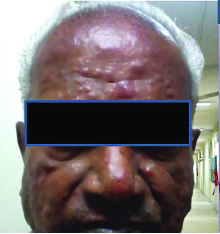
He had no history of pain, itching, fever, loss of sensation, history of injury or history of travel to any endemic area. However, on further questioning, he revealed a history of being investigated multiple times and getting treated at various peripheral centres for Hansen’s disease. The treatment was started based on clinical nature of lesion as biopsy facility was not available at that centre. The patient also underwent Fine Needle Aspiration Cytology (FNAC) at some peripheral centre which was reported as chronic inflammatory lesion only. Based on the FNAC report, a biopsy was done which was reported as leishmaniasis and the patient was treated for the same. Getting tired of the treatment he received without results, the patient decided to visit a higher centre.
On further investigations at our centre, his blood haemogram was normal with ESR of 40 mm at the end of first hour. Blood sugar, liver function and renal function tests were normal. ELISA test for HIV was non-reactive and his CD4 count was normal. ELISA test for kala azar (k-39 antigen) was negative. Serology for Mycobacterium Leprae was negative. Radiograph chest and ultrasonogram abdomen were within normal limits.
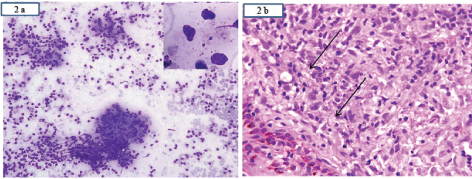
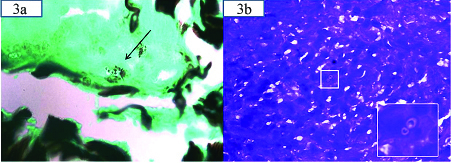
Clinically, a diagnosis of post kala azar dermal leishmaniasis/cutaneous cryptococcosis was considered following which a skin biopsy from the edge of the lesion and slit skin smear was performed. Microscopy revealed few ill-defined granulomas and giant cells both in the epidermis and dermis along with multiple tiny round to oval yeast forms [Table/Fig-2a,b] with a surrounding clear halo suggestive of histoplasmosis. Special stains-silver methenamine [Table/Fig-3a,d] Periodic Acid Schiff (PAS) revealed histoplasma capsulatum lying singly and in clusters. Stain for Fite, Acid Fast Bacilli (AFB), mucicarmine (to rule out cryptococcus) was negative and a diagnosis of primary CHP was given considering the clinical picture, investigations and histopathology. Now, the patient is responding well to the oral fluconazole therapy and is on close follow up.
a) Slit skin smear examination showing multiple granulomas with many extracellular organisms of histoplasma capsulatum (inset; MGG 40X) lying in a background of inflammatory cells (MGG 20X); b) Showing a skin lined tissue with subepithelium showing multiple encapsulated organisms (arrow) lying singly and in clusters surrounded by inflammatory cells (H&E, 40X). (Images left to right)
Showing histoplasma capsulatum in silver methanamine 40X stain and Giemsa stain with inset showing the organism at 60X.
Discussion
Primary CHP is very rare and occurs usually by local trauma or inoculation or inhalation. Its association has been commonly seen in immuocompromised individuals however difficulty arises in patients who are immunocompetent and not hailing from endemic region due to asymptomatic nature of the disease and low clinical suspicion [1]. Primary mode of infection has been postulated as inhalation of conidia (spores) present in soil or suspended in the atmosphere. After inhalation these fungal elements convert into yeast forms which may disseminate systemically [2]. Primary pulmonary histoplasmosis ranges from asymptomatic (most commonly) to mild fever, chest pain and cough which is self-limiting. Biopsy is the most rapid and prime investigative modality to in arrive at a specific diagnosis [3]. Immunocompromised individuals like those who are recipients of solid organ transplants, immunosuppressive drugs, patients having haematolymphoid malignancies and AIDS present often with a severe form of disseminated disease secondary to complication of primary disease or exogenous reinfection or reactivation of latent focus [3]. However, individuals with an intact immune system are able to control and limit the infections and hence the incidence of histoplasmosis in the immunocompetent individuals are rare. Commonly, involved organs in disseminated histoplasmosis are liver, spleen, kidney, lymph node, bone marrow and mucocutaneous tissues. The clinical manifestation in an immunosuppressed is highly variable ranging from what was classically described as a single lesion [4,5] to a polymorphic lesion which closely simulates a variety of infectious and non-infectious diseases [6,7]. The involvement of skin is a result of secondary invasion in disseminated cases [8]. The lesions of CHP are usually missed owing to its polymorphic presentation and a benign course and hence, its low number of reported cases in literature. To overcome the problem of misdiagnosis Wilson proposed a criteria for establishing the diagnosis of CHP [9] which are: 1) History of traumatic inoculation with subsequent development of a lesion (chancre) within three to four weeks at the site of trauma; 2) Isolation in culture medium of the fungus causing the injury; 3) Development of lymphangitis and regional lymphadenopathy; 4) No clinical or laboratory evidence of systemic or pulmonary infection prior; 5) Histoplasmin conversion test from negative to positive with an upward titre. However with increasing knowledge of the disease few modifications have been made namely: 1) Chanceriform injury is not the only manifestation of the disease [8,10–13]; 2) The primary lesion may or may not be accompanied by lymphadenopathy [8,10,12]; 3) The histoplasmin skin test is no longer considered useful, because the reaction does not distinguish between current and past infection, and negative reactions often occur in disseminated disease [13]; 4) In immunosuppressed patients, titers are not reliable and decrease or disappear with the progression of the disease [10–12]. Our case had multiple lesions with no regional lymphadenopathy, no systemic involvement or evidence of immune suppression and is being presented as rare primary CHP, since he had no previous history of trauma or any signs suggesting any healed lesion with no involvement of internal organs and no history of travel to endemic area and is immunocompetent. The source of infection in our case could not be determined and was thought most probably to be by direct inoculation of spores through skin by trivial trauma. The notable feature of this case was the variable presentation in an immunocompetent individual from a non-endemic area should be leading to repeated misdiagnosis and inadequate treatment leading to an awareness of the varied clinical presentation.
Conclusion
Finally, it’s concluded that from a diagnosis point of view CHP can pose a diagnostic challenge as it can present with a variety of skin lesions and mimic lepromatous leprosy and hence, one should keep CHP in the differential diagnosis of nodular lesions even in an immunocompetent individual so as to reduce the morbid stress and unnecessary medications to the patient.
[1]. Vidyanath S, Shameena PM, Nair RG, Disseminated histoplasmosis with oral & cutaneous manifestationsJ Oral Maxillofac Pathol 2013 17(1):139-42. [Google Scholar]
[2]. Harnalikar M, Kharkar V, Khopkar U, Disseminated cutaneous histoplasmosis in an immunocompetent adultIndian J of Dermatology 2012 57(3):206-09. [Google Scholar]
[3]. Rubin H, Furcolow ML, Yates JL, Brasher CA, The course and prognosis of histoplasmosisThe American Journal of Medicine 1959 27:278-88. [Google Scholar]
[4]. Tesh RB, Schneidau JD Jr, Primary cutaneous histoplasmosisThe New England Journal of Medicine 1996 275:597-99. [Google Scholar]
[5]. Tosh FE, Balhuizen J, Yates JL, Brasher CA, Primary cutaneous histoplasmosis. Report of a caseArchives of Internal Medicine 1964 114:118-19. [Google Scholar]
[6]. Cohen PR, Bank DE, Silvers DN, Grossman ME, Cutaneous lesions of disseminated histoplasmosis in human immunodeficiency virus-infected patientsJournal of the American Academy of Dermatology 1990 23:422-28. [Google Scholar]
[7]. Couppie P, Clyti E, Nacher M, Aznar C, Santie-Marie D, Crame B, Acquired immunodeficiency syndrome-related oral and/or cutaneous histoplasmosis: A descriptive and comparative study of 21 cases in French GuianaInternational Journal of Dermatolology 2002 41:571-76. [Google Scholar]
[8]. Saheki MN, Schubach Ade O, Salgueiro Mde M, Conceição-Silva F, Wanke B, Lazera M, Primary cutaneous histoplasmosis: Case report on an immunocompetent patient and review of the literatureJ of Brazilian Society of Tropical Med 2008 41(6):680-82. [Google Scholar]
[9]. Wilson JW, Smith CE, Plunkett OA, Primary cutaneous coccidioidomycosis: The criteria for diagnosis and a report of the caseCalifornia Medicine 1953 79:233-39. [Google Scholar]
[10]. Krunic AL, CARAG H, Medenica MM, Lorincz AL, A case of primary cutaneous histoplasmosis in a patient with diabetes and multi-infarct dementiaJournal of Dermatolology 2002 29:797-802. [Google Scholar]
[11]. Leimann BC, Pizzini CV, Muniz MM, Albuquerque PC, Monteiro PC, Reis RS, Histoplasmosis in a Brazilian center: Clinical forms and laboratory testsRevista Iberoamericana of Micología 2005 22:141-46. [Google Scholar]
[12]. Romano C, Castelli Laurini L, Massai L, Primary cutaneous histoplasmosis in an immunosuppressed patientMycoses 2000 43:151-54. [Google Scholar]
[13]. Cano MV, Hajjeh RA, The epidemiology of histoplasmosis: A reviewSeminars in Respiratory Infections 2001 16:109-18. [Google Scholar]