Burkitt’s Lymphoma in HIV- Positive Child: Diagnostic Ascitic Fluid Cytology
Zeba Choudhary1, Prajwala Gupta2, Madhumitha Udaya Kumar3
1 Senior Resident, Department of Pathology, PGIMER, Dr. Ram Manohar Lohia Hospital, New Delhi, India.
2 Associate Professor, Department of Pathology, PGIMER, Dr. Ram Manohar Lohia Hospital, New Delhi, India.
3 Postgraduate Student, Department of Pathology, PGIMER, Dr. Ram Manohar Lohia Hospital, New Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Prajwala Gupta, C/o Dr. Prajwala Gupta, Room No. 302, OPD Block Dr. Ram Manohar Lohia Hospital, Baba Kharag Singh Marg, New Delhi-110001 India.
E-mail: prajwala2000@yahoo.com
Fluid cytology, Immunodeficiency, Ebstein-barr virus
Dear Editor,
Burkitt’s Lymphoma (BL) is an aggressive, high grade, B-cell lymphoma, that often presents in extranodal sites and is usually seen in young adults and children [1]. It is important to make a rapid diagnosis as BL can present as an oncological emergency due to its extremely rapid doubling time. Since it is common for BL patients to develop serous effusion, cytologic examination of fluid may provide a quick diagnosis in majority of cases.
We present a case of Burkitt’s Lymphoma diagnosed by ascitic fluid cytology in an immunocompromised child.
A 10-year-old male child was admitted to the medical emergency with a five days history of abdominal pain, abdominal distension and fever. There was no history of vomiting, diarrhoea or blood in stool. Patient was a known case of severe immunodeficiency syndrome with HIV positivity. Family history revealed that both the parents were HIV positive. On examination, patient had pallor, moderate ascites, and high grade fever along with mild tender hepatomegaly. There was absence of palpable peripheral lymphadenopathy. Complete blood count with peripheral blood smear examination revealed normocytic normochromic anaemia with mild leucopenia. The biochemical parameters were within normal range. CD4 count analysis revealed 565 cells/mm3. Ultrasonographic examination of the abdomen showed multiple variable sized hypoechoic lesions in the liver, possibly microabscesses and gross ascites. No intra-abdominal lymphadenopathy or mass was noted. Chest radiograph was normal.
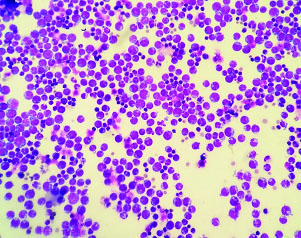
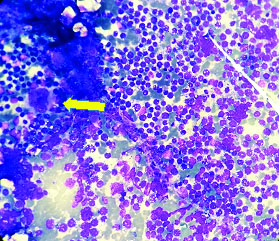
Ascitic fluid was sent for cytological examination. Cytospin smears were prepared and fixed in 95% ethyl alcohol and air dried that were subsequently stained with Papanicolaou and Giemsa stains, respectively. Cytological examination revealed high cellularity with a uniform population of scattered, atypical large lymphoid cells. These cells had eccentric nuclei, some with membrane indentation, multiple prominent nucleoli and scant to moderate amount of basophilic cytoplasm with prominent cytoplasmic vacoulations [Table/Fig-1,2]. Background showed mixed inflammatory cells, few histiocytes, apoptotic debris and mitosis. A diagnosis of Burkitt’s Lymphoma was given. Bone marrow aspiration was performed which revealed infiltration of marrow by these cells with suppressed normal haematopoiesis [Table/Fig-3]. A whole body Computed Tomography (CT) scan and Epstein-barr Virus (EBV) serology were planned but could not be done as the patient deteriorated rapidly within two days of diagnosis and died.
Cytospin preparation showing scattered Burkitt’s lymphoma cells with prominent nucleoli, cytoplasmic basophilia with multiple discrete vacuoles and mitosis (Geimsa; 4X).
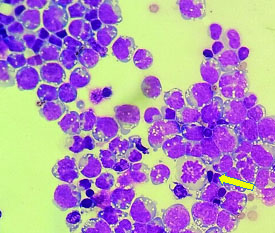
High power view of scattered Burkitt’s lymphoma cells and a mitotic figure (arrow) (Giemsa; 100X).
Bone marrow aspiration smear shows infiltration by Burkitt’s lymphoma cells. A megakaryocyte (arrow) is also seen (Giemsa; 4X).
BL has three clinical variants; endemic, sporadic and immunodeficiency associated. Immunodeficiency associated BL accounts for about 30% of lymphomas in HIV patients and most often are adults [1,2]. Our case illustrates a child with immunodeficiency and positive HIV infection which was vertically transmitted through the mother.
The exact cause and pathophysiologic mechanisms leading to the development of BL are not known but Epstein-Barr virus has been implicated in 25-40% of immunodeficiency-associated cases of BL [2]. HIV infection causes polyclonal activation of B cells in an uncontrolled manner. The genetic instability of EBV positive, aberrantly regulated B cells leads to a risk of c-myc rearrangement and then, to lymphoma. In about 80% of the cases, there is presence of translocation between c-myc gene and the IgH gene t(8;14) and in 20% of cases, there is a translocation between c-myc and kappa or lambda light chain t(2;8) or t(8,22) [1]. The c-myc rearrangement is a crucial event in lymphoma genesis. The Ann Arbor staging system consists of four stages in which the stage 4 refers to central nervous system or bone marrow involvement.
Immunohistochemically, BL cells typically expresses surface IgM, pan B cell antigens including CD19, CD20, CD22 and CD79a and it co-expresses CD10, Bcl-6, CD43 and p53, but are negative for CD5, CD23, Bcl-2, CD138 or TdT [2].
BL typically affects patients with high CD4 T cell counts (>200/mm3) as was seen in our case (565/mm3) which suggests that decreased immunity is not a risk factor in this variant of BL [3].
The quick diagnosis of BL is essential owing to the rapid proliferation of tumour cells so much so that tumour cell lysis may lead to renal failure and sudden death from hyperkalaemia or hypocalcaemia. In our case too, the patient deteriorated rapidly and died due to renal failure and cardiogenic shock. Since it is important to diagnose BL early, cytologic examination of serous fluids can be invaluable. Due to late stage of presentation and very short tumour doubling time, treatment of BL requires urgency. An accurate diagnosis of BL requires the integration of clinical, morphological, immunophenotypic and genetic findings. However, immunophenotypic and genetic studies are laboratory resource intensive and time consuming. A presumptive diagnosis for eminent treatment is usually based on typical clinical and cytomorphological features as was made in our case.
Although the literature on role of cytology in diagnosing BL in an immunocompromised patient is not very much, studies done by Haddad MG et al., and Orem J et al., emphasizes on the role of effusion cytology of BL and outcome of HIV positive BL in paediatric patients, respectively [4,5].
Our case demonstrated the clinical utility of ascitic fluid examination in an immunocompromised paediatric patient with HIV positivity. Although clinically and ultrasonographically, there was no mass lesion or lymphadenopathy but the absence of complete radiological workup in our case could not exclude the same. The recognition of cytological features of Burkitt’s Lymphoma in the ascitic fluid could provide a quick and the only available diagnostic tool in absence of palpable mass and lymphadenopathy and thereby prompting bone marrow aspiration for staging and further management.
[1]. Ferry JA, Burkitt’s lymphoma: clinicopathologic features and differential diagnosisOncologist 2006 11:375-83. [Google Scholar]
[2]. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, WHO Classification of tumours of haematopoietic and lymphoid tissues 2008 4th edLyon, FranceIARC Press:262-64. [Google Scholar]
[3]. Blum KA, Lozanski G, Byrd JC, Adult Burkitt leukemia and lymphomaBlood 2004 104:3009-20. [Google Scholar]
[4]. Haddad MG, Silverman JF, Joshi VV, Geisinger KR, Effusion cytology in Burkitt’s lymphomaDiagnostic cytopathology 1995 12:3-7. [Google Scholar]
[5]. Orem J, Maganda A, Mbidde EK, Weiderpass E, Clinical characteristics and outcome of children with Burkitt lymphoma in Uganda according to HIV infectionPaediatr Blood Cancer 2009 52:455-58. [Google Scholar]