Mature Cystic Teratoma with Co-existent Mucinous Cystadenocarcinoma in the same Ovary-A Diagnostic Dilemma
Sanjeet Roy1, Sramana Mukhopadhayay2, Mayank Gupta3, Anuradha Chandramohan4
1 Assistant Professor, Department of Pathology, Chrisitan Medical College, Vellore, Tamil Nadu, India.
2 Assistant Professor, Department of Pathology, Chrisitan Medical College, Vellore, Tamil Nadu, India.
3 Associate Professor, Department of Pathology, Chrisitan Medical College, Vellore, Tamil Nadu, India.
4 Associate Professor, Department of Diagnostic Radiology and Imaging, Chrisitan Medical College, Vellore, Tamil Nadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Sramana Mukhopadhayay Assistant Professor, Department of Pathology, Chrisitan Medical College, Vellore-632004, Tamil Nadu, India.
E-mail: drsramana@gmail.com
Mature cystic teratoma co-existing with a mucinous cystadenocarcinoma is an infrequently encountered entity with only a handful of cases reported till date. The possibilities in such a case are either a malignant transformation of a benign teratoma into adenocarcinoma or a collision tumor between a mature cystic teratoma and a mucinous tumour of either a primary ovarian surface epithelial-stromal origin or a secondary from a primary gastrointestinal tract tumour. The importance of distinguishing between the two entities has significant bearing on subsequent therapeutic management.
We report a rare case of a mature cystic teratoma co-existing with a mucinous cystadenocarcinoma in the same ovary in a 44-year-old lady. Contrast Enhanced Computed Tomography (CECT) imaging of the left ovarian mass was suggestive of a teratoma but an intra-operative frozen section examination was reported as an adenocarcinoma with a cystic teratoma. Gross examination of the surgical specimen revealed a dermoid cyst with another multi-septated cystic lesion containing mucoid material. Histopathological examination showed a mature cystic teratoma and an associated well differentiated mucinous cystadenocarcinoma. The latter displayed a CK7-ve/CK20+ve immunoprofile. In absence of clinical, biochemical or radiological findings of a primary lower gastrointestinal tract tumour, the immunoprofile suggested the possibility of adenocarcinomatous transformation in a benign teratoma.
Mucinous neoplasm, Collision tumour, Malignant transformation
Case Report
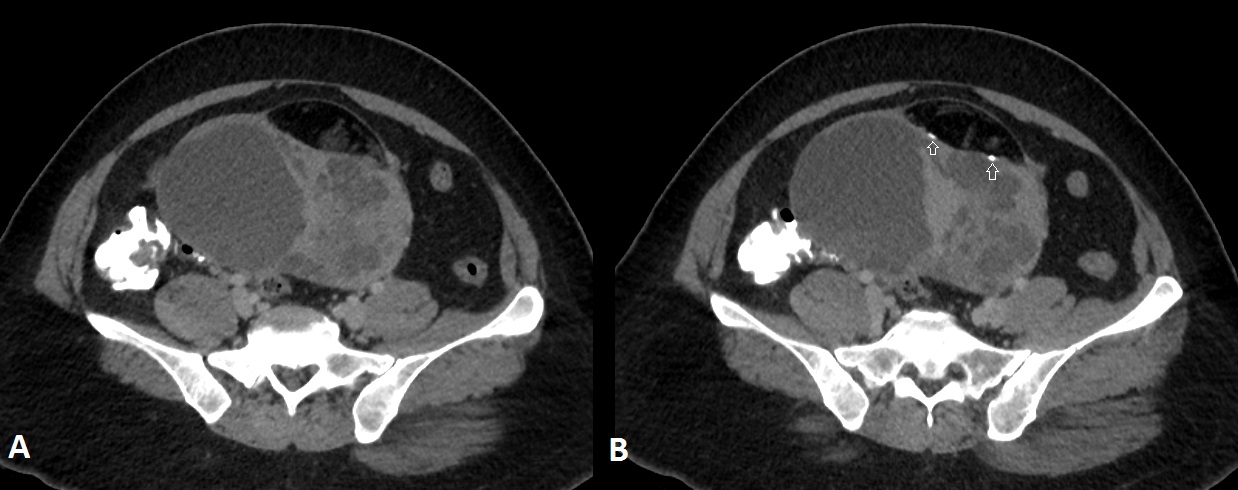
A 44-year-old perimenopausal lady on evaluation for irregular menstrual cycles was found to have a palpable cystic mass 8x10cm in the lower abdomen. A computed tomography of the abdomen and pelvis [Table/Fig-1a,b] showed a ~ 10x14x9.5cm sized well defined mixed density mass containing solid, cystic and fat density components and eccentric nodular foci of calcification arising from the left adnexa [Table/Fig-2]. Since, there was heterogeneous solid component clearly to one side of the lesion and mixed density component to the other side, possibilities of immature teratoma and collision tumour was suggested based on CT findings. There were no other primary malignancies from gastrointestinal tract, breast or any other organs. Pre-operative CA125 was 8.94U/L (Reference range <35U/L) and CA19-9 was 8.36U/L (Reference range 0-33U/L). A staging laprotomy with total abdominal hysterectomy and bilateral salpingo-oophorectomy was done. Since, suspicious solid areas were seen in the ovarian cyst intra-operatively, frozen section was sent to exclude malignancy. Frozen section revealed adenocarcinoma with features of cystic teratoma. In view of this, pelvic lymphadenectomy, omentectomy and appendicectomy were also done at the same sitting.
Axial sections of Contrast Enhanced Computed Tomography (CECT) through the lower abdomen shows a complex cystic mass with thick internal septations and enhancing solid areas in the right adnexa. There is an eccentric well defined lentiform fat density component on the anterior aspect of the mass with nodular calcifications (arrows).
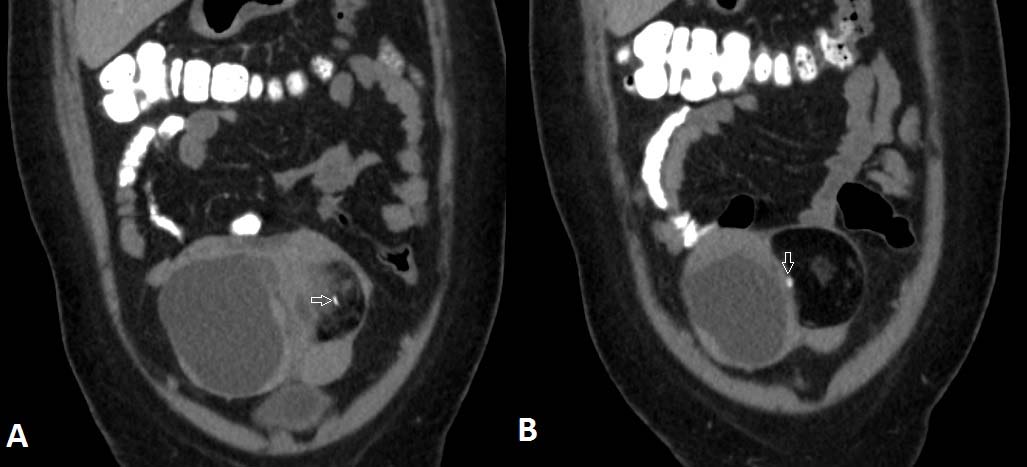
Coronal re-constructions of the CECT of the same patient show the relationship between the fat density lesion and the complex cystic mass. Calcification is shown with arrows.
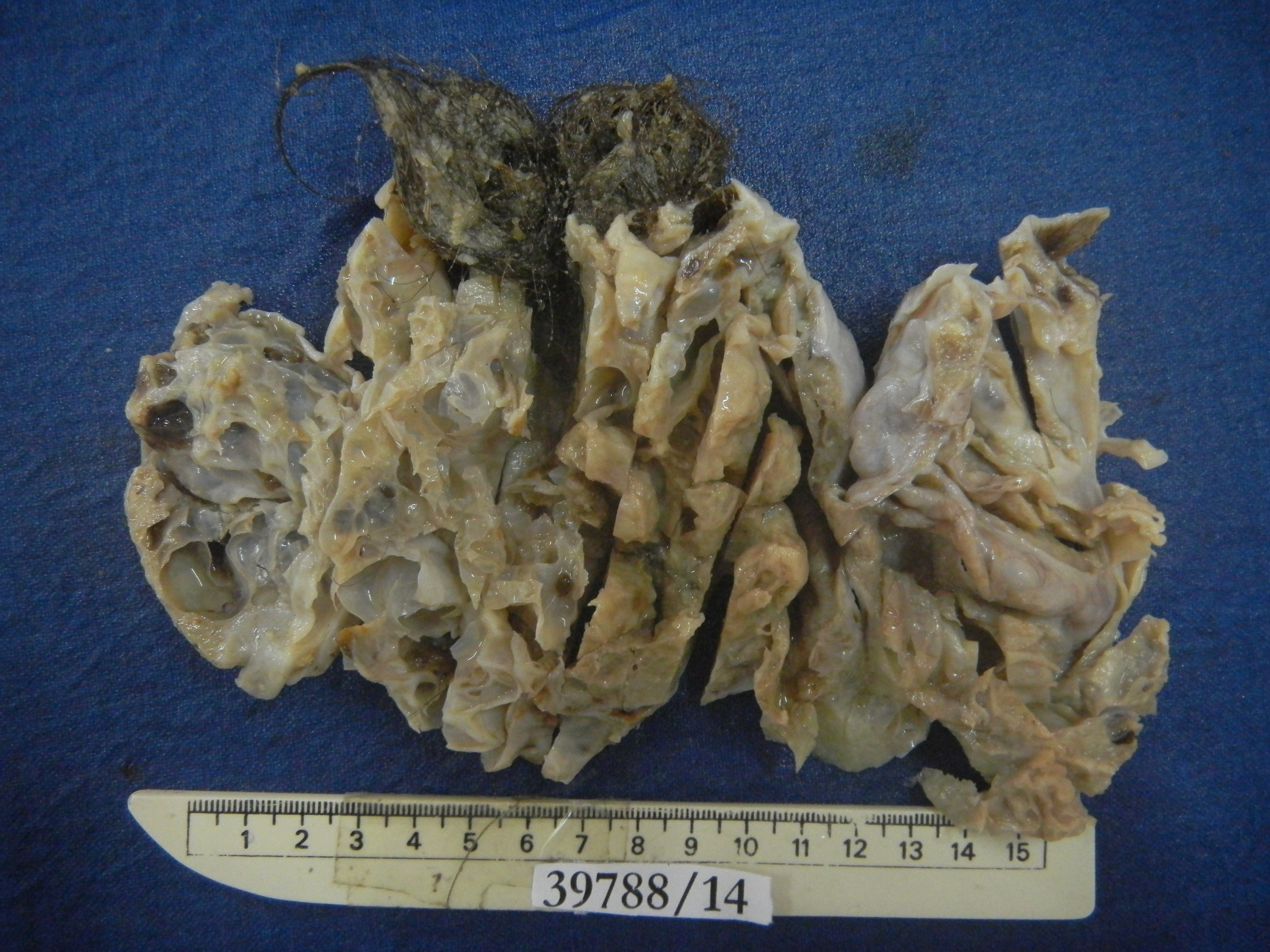
Gross examination of the surgical specimen revealed a solid cystic mass involving the left ovary measuring 11x9.5x5cm with a smooth external surface and no capsular breach. The cut surface showed two well demarcated lesions, one containing tufts of hair and grumous pultaceous material and adjacent to it another solid cystic multi-loculated lesion with cysts ranging from 0.1cm to 3.5cm and containing mucoid material [Table/Fig-3]. There were no papillary excrescences identified. The uterus, cervix, right ovary and fallopian tube and appendix appeared grossly un-remarkable.
Cut section of left ovarian mass showing a cystic teratoma with tufts of hair and adjacent multi-loculated solid cystic lesion.
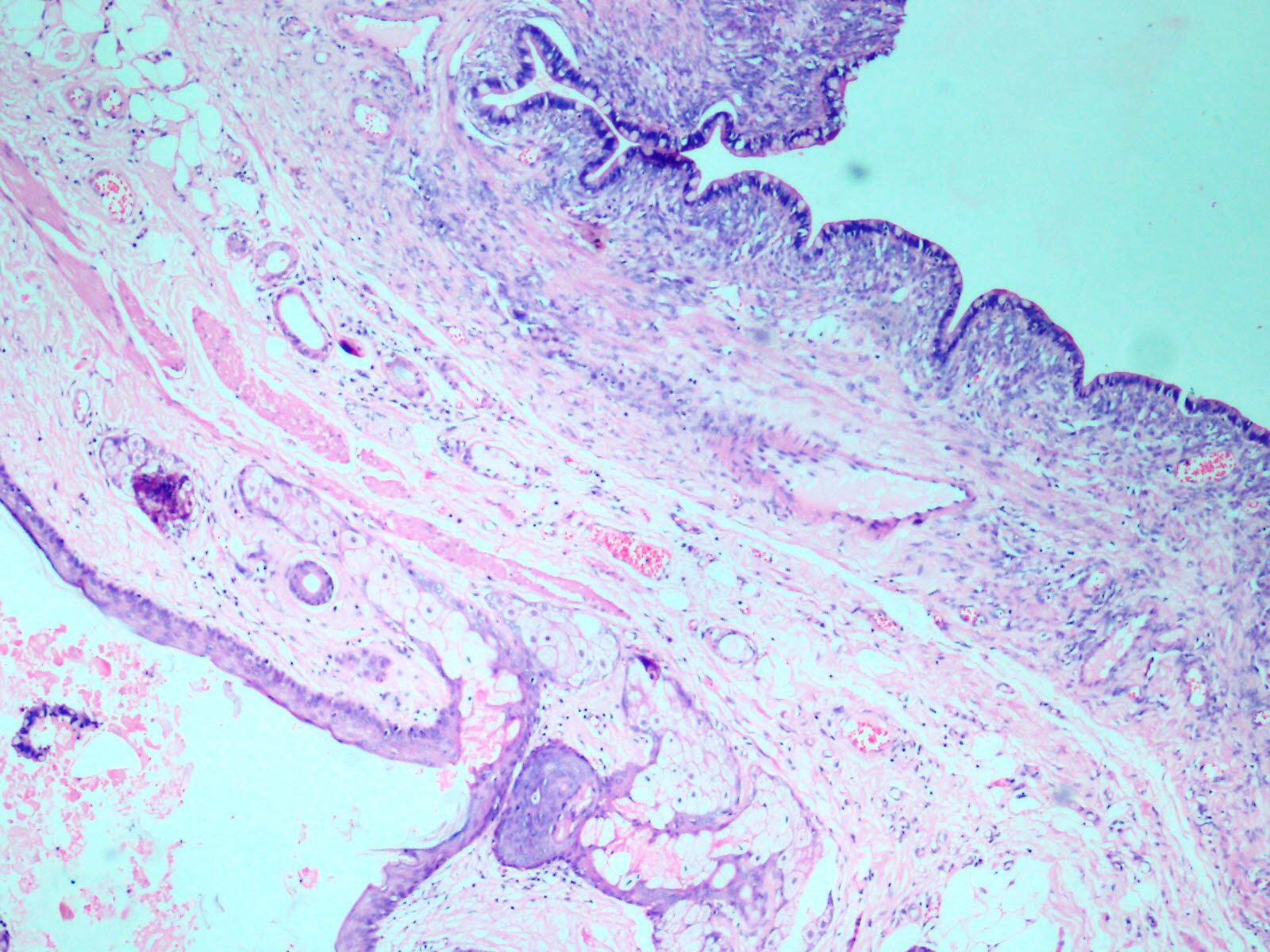
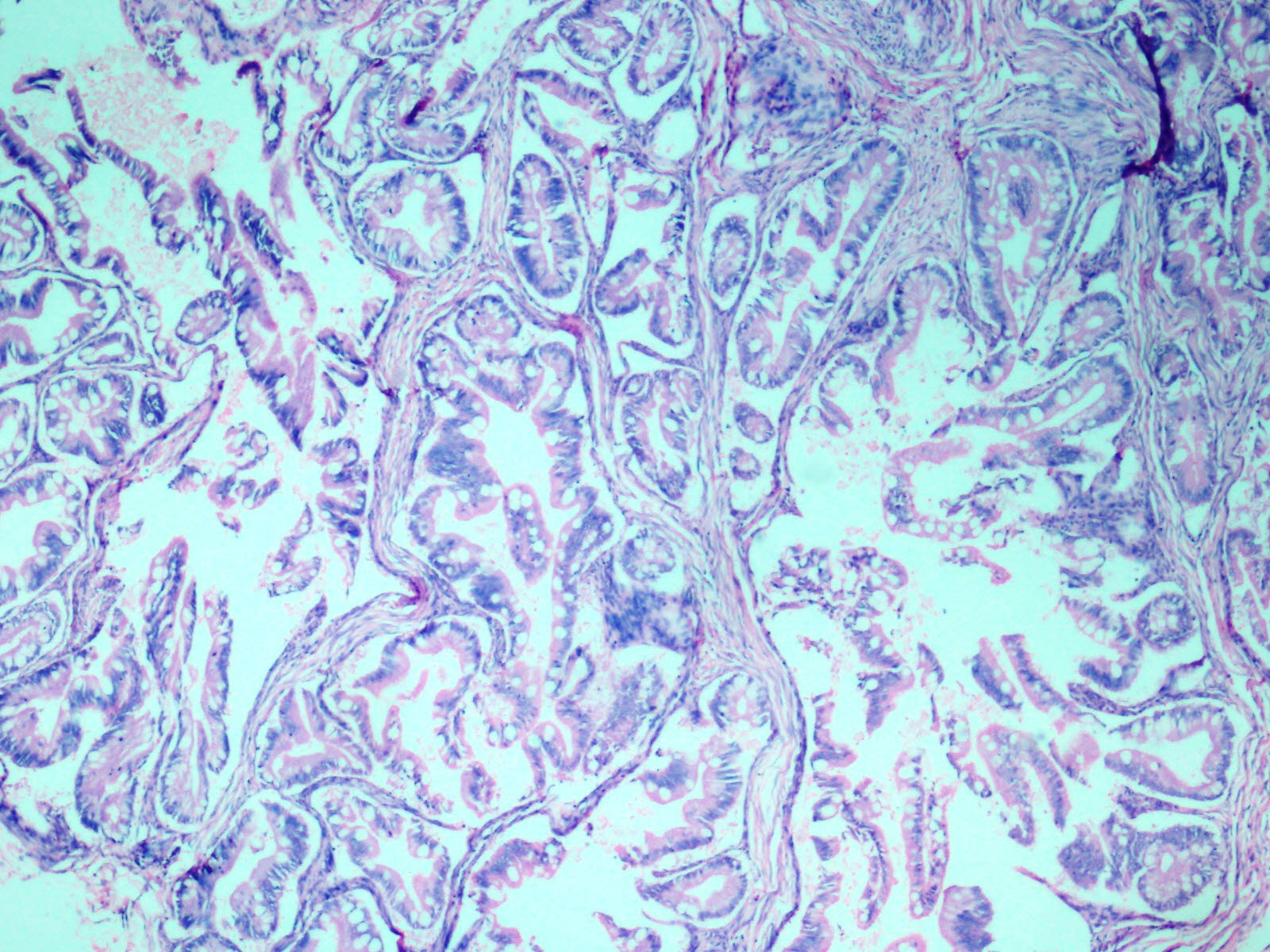
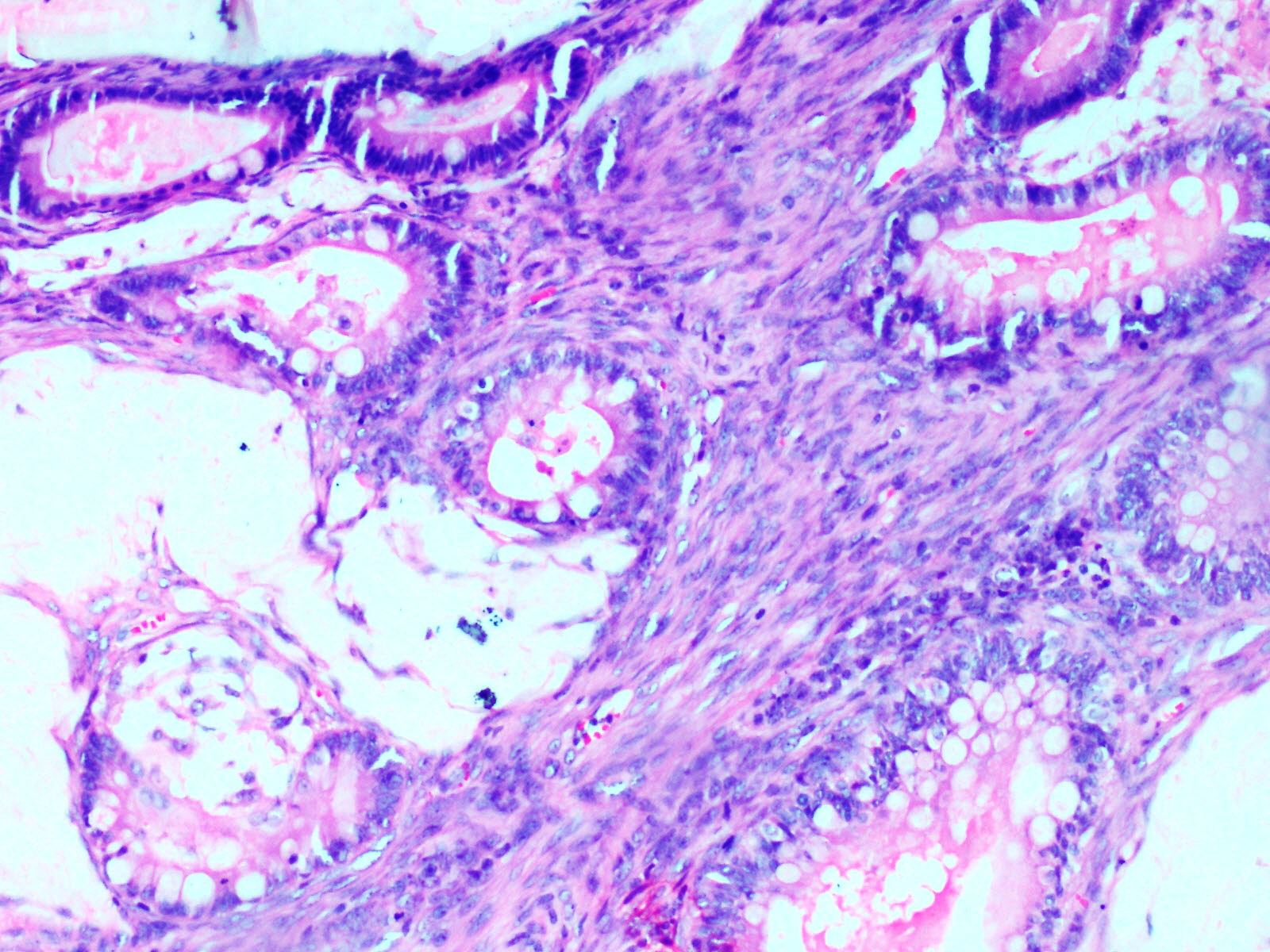
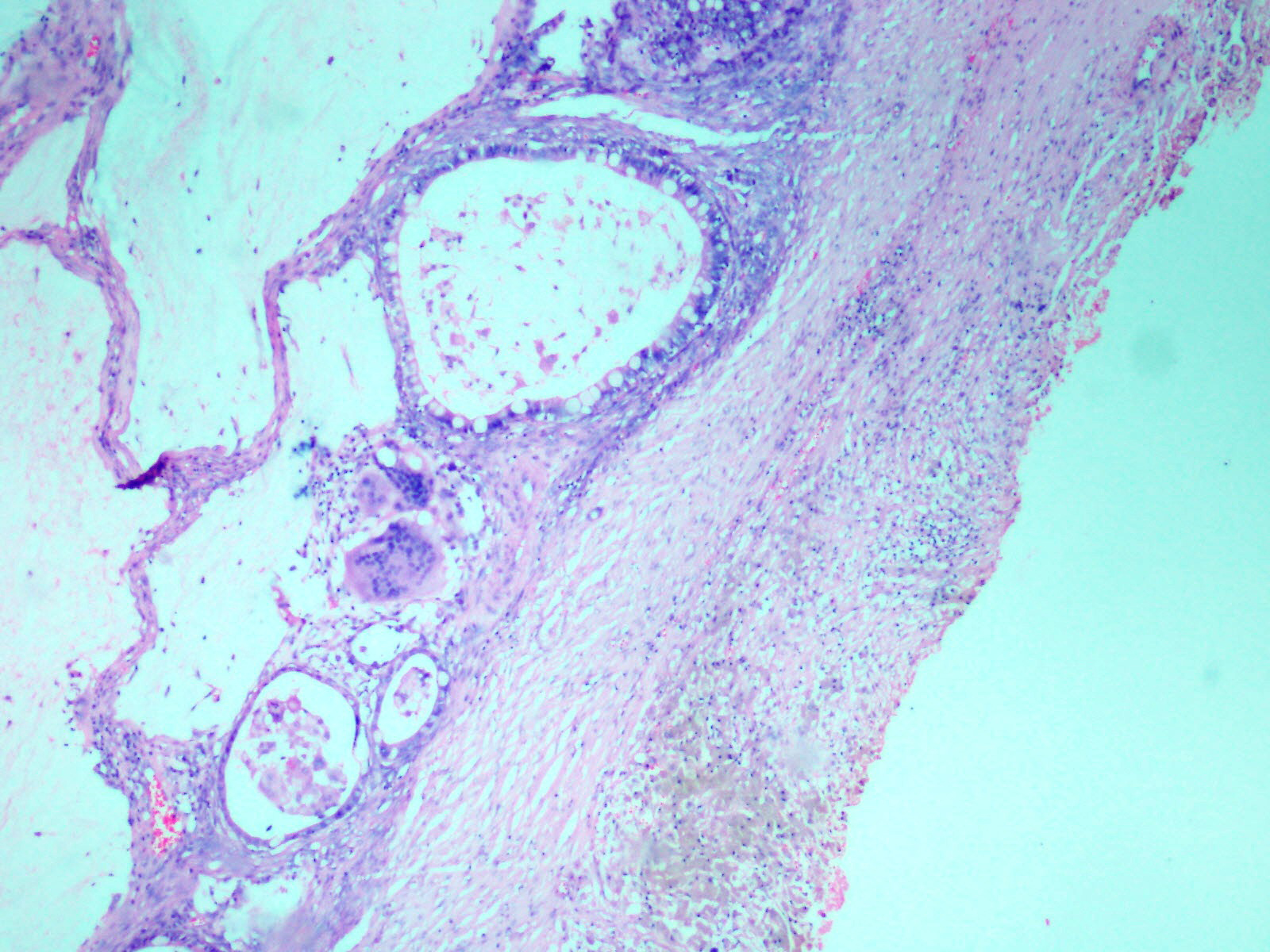
Histopathological examination of the ovarian mass showed two distinct cystic lesions [Table/Fig-4], one of which was a mature cystic teratoma displaying derivatives from all three germ layers with presence of many hair shafts towards the luminal surface, skin with adnexal elements and hair follicles, pigment laden melanophages, cluster of mature adipocytes, skeletal muscle and also benign glandular elements lined by intestinal type epithelium. There were no immature elements. The second component was composed of numerous closely packed complex glands lined by intestinal type epithelium containing goblet cells and exhibiting moderate nuclear pleomorphism, stratification and focal loss of polarity [Table/Fig-5] with infiltration of the neoplastic glandular elements into the ovarian parenchyma [Table/Fig-6]. Foci of necrosis and features of pseudomyxoma ovarii with pools of extracellular mucin pools within the ovarian stroma were also present [Table/Fig-7]. The specimen was extensively sampled but on microscopic examination no distinct rim of intervening normal ovarian parenchyma clearly demarcating the two lesions was seen. An intermediate transitional zone seemed to be present within the two tumours making it difficult to differentiate on histology alone as collision tumour or a true mixed tumour. The uterus, cervix, right ovary and bilateral fallopian tubes did not reveal any significant lesion. The appendix showed lymphoid hyperplasia and no evidence of malignancy.
Wall of cyst with adnexal structures and intestinal epithelium. (H&E 10X).
Complex and crowded glandular structures lined by intestinal type epithelium with prominent goblet cells. (H&E, 20X).
Stromal invasion by adenocarcinoma component. (H&E, 40X).
Presence of mucin pools with admixed goblet cells and accompanying giant cell reaction. (H&E, 20X).
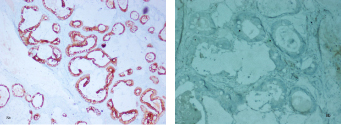
On immunohistochemistry the neoplastic glands showed diffuse positivity for CK20 and were negative for CK7 [Table/Fig-8a,b]. The final diagnosis offered was a mature cystic teratoma and co-existing well differentiated mucinous cystadenocarcinoma of the left ovary, FIGO stage Ia.
The tumour cells are diffusely positive for CK20(a) and negative for CK 7 (20X) (b).
Post-operatively the patient was followed up for 22 months. There was no evidence of tumour recurrence and serum CA125 was 3.55 U/L.
Discussion
Mature cystic teratomas are tumours of children and young adults but can be encountered at any age. Malignant transformation in the teratomatous elements is reported in only 1-2% of cases of a benign teratoma [1,2] of which a squamous cell carcinoma is the most common malignancy to arise [2,3]. Adenocarcinoma forms only 7% of the cases reported with malignant transformation [4]. In the present case a well differentiated mucinous cyst adenocarcinoma was seen in association with a mature cystic teratoma.
2 to 11% mature cystic teratomas have been found to be associated with mucinous tumours [5]. These teratoma associated mucinous tumours can be cystadenomatous, borderline or frankly malignant though the latter combination is distinctly uncommon. Imaging findings in the present case were suggestive of a collision tumour. Various combinations of a teratoma with granulosa cell tumour, serous adenocarcinoma and steroid cell tumours have been previously documented forming collision tumours in the ovary [6]. Absence of histological admixture at the interface between the two tumour entities is the foremost criterion for defining a collision tumour [7]. However, histological examination in the present case did not show a distinct rim of intervening normal ovarian parenchyma demarcating the two tumour entities to establish a diagnosis of a collision tumour. Most primary ovarian mucinous tumours of surface epithelial-stromal origin show diffuse CK7 positivity with variable expression of CK20 [8]. The immunohistochemical analysis in the present case however showed a CK20+ve/CK7-ve immunoprofile within the mucinous cystadenocarcinoma component. Such an immunoprofile can be explained by either a metastases from a lower gastrointestinal tract primary such as in the colorectum or the appendix or due to a mucinous neoplasm arising from the lower gastrointestinal tract type tissue present in the ovarian teratoma itself. Extensive pre-operative clinical and radiological evaluation in the present case did not reveal a second malignant neoplasm in either the upper or the lower gastrointestinal tract or any other site or organ. Serological evaluation showed normal CA19-9 and CA-125 levels. The surgical specimen of the appendix was also free of tumour. Therefore, the diagnosis was in favour of a mucinous cystadenocarcinoma of the intestinal type arising from a mature cystic teratoma. A post-operative follow-up for 22 months has not shown any recurrence or revealed any second neoplasm in the patient corroborating our diagnosis.
Definite evidence in favour of a primary aetiology rather than a metastasis are lacking in previously published literature on similar cases [9–12]. In a recent report of mucinous adenocarcinoma arising in a mature cystic teratoma, the adenocarcinomatous component was concurred as a primary on the absence of recurrence over a follow-up period of 6 months and CK7 positivity. However, in the present case the follow-up period was more than a year. Primary mucinous neoplasm in the appendix was excluded by examination of the resected surgical specimen. Radiological and serological evidence of a second tumour elsewhere was also not found. Moreover, histopathological examination of the ovarian mass depicting an intermediate transitional zone between the benign and malignant components and immunohistochemical analysis with CK20+ve/CK7-ve profile were robust evidences in favour of our diagnosis.
In view of the wide variety of ectodermal, endodermal and mesodermal tissues that a teratoma can be associated with, it is not surprising that an additional neoplasm can supervene in the same ovary. A study done by Vang et al., on 44 mucinous ovarian tumours associated with mature cystic teratoma revealed only 6 cases with carcinomatous transformation, all 6 of which had pseudomyxoma ovarii [8]. The presence of pseudomyxoma ovarii was significantly associated with a CK20+ve/CK7-ve immunoprofile in his study. A CK20+ve/CK7-ve immunoprofile can also suggest a secondary ovarian tumour from a primary lower gastrointestinal tract malignancy. However, presence of adjunct features of metastasis such as bilaterality of tumour involvement, extra pelvic spread, multinodularity and hilus involvement [8] can be important clues in addition to a detailed clinical evaluation and follow-up failing to identify a non-ovarian source of mucinous neoplasm elsewhere.
Conclusion
This case highlights the importance of meticulous recording of the gross features, extensive sampling, histopathological examination including immunohistochemistry and supportive clinical and radiological findings to arrive at a correct diagnosis in case of unconventional tumours.
Ethical concerns: None.
[1]. Ayhan A, Bukulmez O, Genc C, Karamursel BS, Ayhan A, Mature cystic teratomas of the ovary: case series from one institution over 34 yearsEur J Obstet Gynecol Reprod Biol 2000 88(2):153-57. [Google Scholar]
[2]. Hirakawa T, Tsuneyoshi M, Enjoji M, Squamous cell carcinoma arising in mature cystic teratoma of the ovary. Clinicopathologic and topographic analysisAm J Surg Pathol 1989 13(5):397-405. [Google Scholar]
[3]. Krumerman MS, Chung A, Squamous carcinoma arising in benign cystic teratoma of the ovary: a report of four cases and review of the literatureCancer 1977 39(3):1237-42. [Google Scholar]
[4]. Güney M, Oral B, Demir F, Ozsoy M, Kapucuoğlu N, Mucinous adenocarcinoma arising from the gastrointestinal epithelium in benign cystic teratoma of the ovary—case reportEur J Gynaecol Oncol 2006 27(3):304-06. [Google Scholar]
[5]. Ueda G, Fujita M, Ogawa H, Sawada M, Inoue M, Tanizawa O, Adenocarcinoma in a benign cystic teratoma of the ovary: report of a case with a long survival periodGynecol Oncol 1993 48(2):259-63. [Google Scholar]
[6]. Bostanci MS, Yildirim OK, Yildirim G, Bakacak M, Ekinci ID, Collision tumor: Dermoid cysts and mucinous cystadenoma in the same ovary and a review of the literatureObstet Gynecol Cases Rev 2015 2:31 [Google Scholar]
[7]. Singh AK, Singh M, Collision tumours of ovary: A very rare case seriesJ Clin Diagn Res JCDR 2014 8(11):FD14-6. [Google Scholar]
[8]. Vang R, Gown AM, Zhao C, Barry TS, Isacson C, Richardson MS, Ovarian mucinous tumors associated with mature cystic teratomas: morphologic and immunohistochemical analysis identifies a subset of potential teratomatous origin that shares features of lower gastrointestinal tract mucinous tumors more commonly encountered as secondary tumors in the ovaryAm J Surg Pathol 2007 31(6):854-69. [Google Scholar]
[9]. Miyasaka A, Nishikawa T, Kozawa E, Yasuda M, Fujiwara K, Hasegawa K, Advanced mucinous adenocarcinoma arising from a mature cystic teratoma: A case report and literature reviewCase Rep Oncol 2016 9(2):331-37. [Google Scholar]
[10]. Pintea MD, Mucinous cystadenoma arising in a mature cystic teratoma in a 25-Year-Old PatientCase Rep Obstet Gynecol 2014 2014:1-2. [Google Scholar]
[11]. Mandal S, Kawatra V, Khurana N, Mucinous cystadenocarcinoma arising in mature cystic teratoma ovary and associated pseudomyxoma peritonei: report of a caseArch Gynecol Obstet 2008 278(3):265-67. [Google Scholar]
[12]. Ray S, Anuradha D, Barui G, Karmakar R, Sinha A, Bhattacharya A, Co-existence of a mature teratoma and mucinous cystadenocarcinoma in the same ovary—a case reportIndian J Pathol Microbiol 2006 49(3):420 [Google Scholar]