Solitary Thyroid Nodule (STN) is defined as a palpable discrete swelling within an otherwise apparently normal thyroid gland. STNs are diagnosed in 4%–8% of adults by palpation and in 13%–67% of adults when ultrasound detection is used [1]. STN has provoked increased concern owing to higher incidence of malignancy in it as compared to Multinodular Goiter (MNG) [2]. Thyroid cancers occur in approximately 5% of all thyroid nodules independent of their size [1]. It is necessary to differentiate patients with benign STN from malignant ones for early treatment strategy and better patient management.
Characteristic nuclear changes are necessary for the diagnosis of papillary thyroid carcinoma (PTC), however when present focally along with papillary structures they cause diagnostic dilemma in distinguishing it from other thyroid lesions [3]. Currently, the standard diagnosis depends on the histomorphologic examination of routine Haematoxylin and Eosin (H&E) stained slides, but interobserver or intraobserver disagreements in the diagnosis of papillary and follicular thyroid lesions are well known and documented in literature [4].
Various newer Immunohistochemistry (IHC) markers are being described and validated for differentiating benign STN from malignant ones and Follicular Variant of Papillary Carcinoma (FVPTC) from Follicular Carcinoma (FCa) or Follicular Adenoma (FA) [5]. In the present study, we analysed IHC markers (CK-19, CD-56, p53, Ki-67) to differentiate between benign and malignant surgically resected STN along with their utility in the identification of PTC.
Materials and Methods
This cross sectional study consisted of surgically resected thyroid specimens of 160 patients with STN received in the Department of Pathology over 4 years. Records of clinical, ultrasonography (USG) and laboratory data was retrieved and analysed. STN cases diagnosed on USG, clinically and on gross thyroid specimen examination were studied which included dominant/hyperplastic nodule in MNG, colloid nodule, FA and its variants and FCa, PTC and its variants. Thyroid lesions with multiple nodules or diffuse hyperplasia and tumours/lesions which were not of follicular epithelial cell origin were excluded from the present study.
The gross and histopathological features of each case were evaluated independently by two pathologists before arriving at the final diagnosis. Tumour staging was determined according to the TNM staging [6]. Histologic grading was done based on the presence/absence of nuclear atypia, mitoses, tumour necrosis, and vascular invasion [7,8].
A technique of manual tissue array was employed for all the cases subjected for IHC [9]. The primary antibodies used were CK-19 (Clone RCK108; Dako), CD-56 (Clone 1B6; Novocastra), p53 (Clone DO-7; Novocastra) and Ki-67 (Clone MM-1; Novocastra). Negative control (without adding primary antibody) was included in all batches. Section from skin was used as positive control for CK-19. A section from tonsil was used as positive control for CD-56 and Ki-67. Section from breast cancer tissue, which previously showed unequivocal strong immunoreactivity, was used as positive control for p53. Sections were examined under High Power.
CK-19
Semiquantitative scoring of CK-19 was done according to percentage of cells showing membranous positivity [10,11]. Score of 1+,2+,3+ and 4+ was assigned for <5%, 5 – 25 %, 25 – 75% and >75% positively stained cells.
CD-56
CD-56 expression in ≥10% of cells showing membranous positivity was considered as positive and <10% as negative [12,13].
Ki-67
The nuclear staining for Ki-67 was scored by calculating the number of positively stained tumour nuclei in 1000 tumour cells in the hot spot of tumour. The Ki-67 Labeling index (Ki-67LI) (expressed as percentage of positively stained cells per 100 follicular epithelial cell) was recorded [14,15]. The cases which showed <5% nuclear positivity were taken as p53 negative whereas those which showed ≥5% nuclear positivity were considered as positive [16].
Statistical Analysis
The statistical software named Primer software Version 5.0 (manufactured by McGraw-Hill) was used for analysing the data. The groups were compared using the Pearson’s Chi-square test (PCT) or Fisher-Exact-test (FET). ANOVA test was used to compare the difference in mean between multiple groups and t-test was used to compare the difference in mean between two groups. The p-value of 0.05 or less was considered statistically significant.
Results
Distribution of STN cases in this study is shown in [Table/Fig-1]. The study cases comprised of 83.12% (133/160) females and 17.88% (27/160) males. STN was mainly seen in the age group of 21-40 years (98/160, 61.25%). Peak occurrence of malignancy in STN was seen in the 5th decade (19/68, 27.94%). Highest percentage of PTC (32/61, 52.46%) was seen between 31-50 years whereas that of FCa (3/7, 42.86%) was seen between 41-50 years age group. There were 7 cases in 2nd decade of life, out of which 3 were benign and 4 were malignant. These 4 malignant cases were diagnosed as PTC.
Distribution of solitary thyroid nodule cases in this study based on histopathological examination.
| Study group | Total no of cases | Percentage (%) |
|---|
| Non-neoplastic lesions | 68 | 42.5 |
| Benign neoplasms | 24 | 15 |
| Malignanttumours | FCa | 07 | 4.38 |
| UTPTC | 36 | 22.5 |
| FVPTC | 25 | 15.62 |
| Total | 160 | 100 |
STN-solitary thyroid nodule, FCa-follicular carcinoma, UTPTC-usual type papillary thyroid carcinoma FVPTC-follicular variant of papillary thyroid carcinoma.
Majority of the patients were euthyroid (141/160, 88.13%) followed by hypothyroidi (17/160, 10.63%) and hyperthyroid (2/160, 1.24%). Hemithyroidectomy was performed in 85.63% (137/160,) cases. Rest had undergone total thyroidectomy, largely performed in cases of malignant tumours.
The STN diameter ranged from 1 to 12 cm. The smallest lesion (1cm) in our study was due to non-neoplastic lesions and the largest lesion (12cm) was caused by FCa. Majority of malignant tumours (39/68, 57.36%) were >4cm and non-neoplastic lesions (28/68, 41.20%) were ≤2 cm in their largest dimension.
The lymph node metastasis in PTC cases was seen in 19.67% (12/61) cases which included 8 cases of PTC and 4 cases of FVPTC. None of the FCa cases showed metastasis to lymph node. Out of 68 malignant cases, one case of FVPTC showed extra thyroidal extension. 5/7(71.43%) cases of FCa were of grade 2 at the time of presentation. They showed evidence of vascular invasion and 3 cases showed nuclear atypia. 2/7 cases (28.57%) were in grade 1 at the time of diagnosis. 8.20% (5/61 cases) of PTC cases were of grade 2 at the time of diagnosis which included 4 cases of usual type PTC (UTPTC) and 1 case of FVPTC. Vascular invasion was seen in this FVPTC case. 3 cases of UTPTC showed lymphovascular invasion. All the grade 2 PTC cases showed mild to moderate nuclear atypia.
Amongst malignant tumours, 24.6% (15/68) of PTC cases and 42.86% (3/68) of FCa cases were in stage III at the time of diagnosis. 70.49% (43/61) of PTC and 42.86% (3/7) of FCa cases were in stage I at the time of diagnosis. Only 1/61 (1.64%) cases of PTC was in stage IV at the time of diagnosis. This case was FVPTC.
Semiquantitative expression of CK-19 in non-neoplastic lesions, FA, FCa and PTC cases is given in [Table/Fig-2,3 and 4]. Taking 4+ as the cut off value for CK-19 positivity [11] aim as detecting PTC versus non PTC cases in the study, it was noticed that 51/61 cases of PTC were positive for CK-19 and the cases belonging to non-PTC group showed 100% (99/99) negativity. There was no statistically significant difference (χ2=1.263; p=0.261) between the CK-19 expression of UTPTC and FVPTC. Clinico-pathological parameters such as male: female ratio, mean age, mean tumour size, tumour staging, histologic grading [Table/Fig-5] of CK-19 positive and negative cases were studied. The mean age at diagnosis of CK-19 positive PTC cases was 38.86y±8.81 and that of CK-19 negative PTC cases was 42.7y±3.698, however the difference between the mean ages was statistically insignificant. (p=0.183). The mean tumour size of CK-19 positive PTC cases was 4.78cm±8.81 and that of CK-19 negative PTC cases was 6.12cm±3.698, however the difference between the mean tumour sizes was statistically insignificant. (p=0.640) CK-19 showed 83.61% sensitivity, 100% specificity and 100% positive predictive value for the diagnosis of PTC.
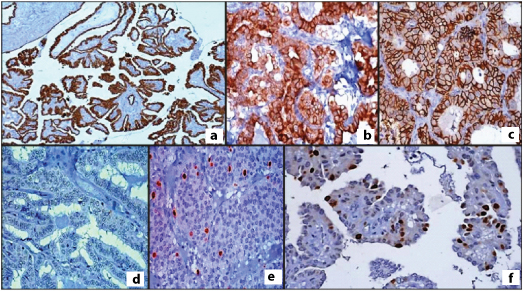
CK-19 membranous positivity in: a) papillary thyroid carcinoma (10X); and b) follicular variant of papillary thyroid carcinoma (40X); c) CD-56 membranous positivity in follicular adenoma (40X); and d) Absence of CD-56 expression in papillary thyroid carcinoma (40X); e) p53 positive expression in follicular carcinoma (40X); and f) Ki-67 positivity in papillary thyroid carcinoma (40X).
Semiquantitative expression of CK-19 in Non-neoplastic lesions, Follicular adenoma, FCa and PTC cases.
| CK-19 score | Non- neoplasticlesions (n=68)% | Follicular adenoma(n = 24)% | FCa(n = 07)% | Papillary thyroid carcinoma (n= 61) | Total PTCcases (n= 61)% |
|---|
| UTPTC (n = 36)% | FVPTC (n=25)% |
|---|
| 1+ (<5%) | 45 (66.18%) | 12(50%) | 04(57.14%) | 01(2.78%) | - | 01(1.63%) |
| 2+ (5-25%) | 18(26.47%) | 10(41.67%) | 03(42.86%) | 01(2.78%) | 01(4%) | 02(3.28%) |
| 3+ (25-75%) | 05(7.35%) | 02(8.33%) | 0 | 06(16.66%) | 01(4%) | 07(11.48%) |
| 4+ (>75%) | 0 | 0 | 0 | 28(77.78%) | 23(92%) | 51(83.61%) |
FCa-follicular carcinoma, UTPTC-usual type papillary thyroid carcinoma FVPTC-follicular variant of papillary thyroid carcinoma, PTC-papillary thyroid carcinoma
CK-19, CD-56 and p53 expression in benign and malignant STN lesions.
| IHCmarkers | Expression | Benign | Malignant |
|---|
| Non neoplastic(n = 68) % | Neoplastic(n=24)% | Total(n=92)% | FCa (n=07)% | PTC (n = 61) | Total (n=68)% |
|---|
| UTPTC(n=36)% | FVPTC(n=25)% |
|---|
| CK-19 | Positive | 0 | 0 | 0 | 0 | 28(77.78%) | 23(92%) | 51(75%) |
| Negative | 68(100%) | 24(100%) | 92(100%) | 07(100%) | 08(22.22%) | 02(8%) | 17(25%) |
| CD-56 | Positive | 59 (86.76%) | 20(83.33%) | 79(85.87%) | 06(85.71%) | 08 (22.22%) | 01 (4%) | 15(22.06%) |
| Negative | 09 (13.24%) | 04(16.67%) | 13(14.13%) | 01(14.29%) | 28 (77.78%) | 24 (96%) | 53 (77.94%) |
| p53 | Positive | 12 (17.65%) | 15 (62.5%) | 27 (29.35%) | 06 (85.71%) | 31 (86.11%) | 21 (84%) | 58 (85.29%) |
| Negative | 56 (82.35%) | 09 (37.5%) | 65 (70.65%) | 01 (14.29%) | 05 (13.89%) | 04(16%) | 10 (14.71%) |
STN-Solitary thyroid nodule, IHC-immunohistochemistry, FCa-follicular carcinoma, PTC- papillary thyroid carcinoma, UTPTC-usual type papillary thyroid carcinoma FVPTC-follicular variant of papillary thyroid carcinoma.
Comparison of CK-19 positive PTC versus CK-19 negative PTC and CD-56 positive PTC versus CD-56 negative PTC.
| Parameters | CK-19 positivePTC cases(n = 51)% | CK-19 negativePTC cases(n = 10)% | p-value | CD-56 positivePTC cases(n=9)% | CD-56 negativePTC cases(n=52) | p-value |
|---|
| Female | 40 | 8 | | 7 | 40 | |
| Male | 11 | 2 | 2 | 12 | |
| Mean age at diagnosis (yrs) | 38.86 | 42.7 | p=0.183 | 48.11 | 38 | p=<0.01 |
| Mean tumour size at diagnosis (cms) | 4.78 | 6.12 | p=0.640 | 5.97 | 4.83 | p=0.507 |
| TNM staging Stage I | 37(72.55%) | 06(60%) | χ2=1.444;p=0.486 | 5 (55.56%) | 38 (73.08%) | χ2=2.016;p=0.365 |
| Stage II | 02(3.92%) | 0 | 0 | 02 (3.85%) |
| Stage III | 12(23.53%) | 04(40%) | 04 | 12 (23.08%) |
| Stage IV | 0 | 0 | 0 | 0 |
| GradingGrade I | 45 (88.24%) | 10(100%) | p=0.577 | 6 (66.67%) | 49 (94.23%) | p=0.037 |
| Grade II | 06(11.76%) | 0 | 3 (3.33%) | 3 (5.77%) |
PTC- papillary thyroid carcinoma.
A total of 52/61 (85.25%) cases of PTC showed absent/weak [Table/Fig-4] and 9/61(14.75%) cases showed mild to moderate CD-56 expression [Table/Fig-2c,d]. In non-PTC group 85/99 (85.86%) cases showed strong diffuse membranous positivity and 14/99 (14.14%) cases showed only focal/weak CD-56 immunoreactivity. This difference was found to be highly statistically significant (χ2=75.835, p<0.001). 79/92 (85.87%) non-neoplastic and benign neoplastic cases and 6/7 (85.71%) FCa cases showed strong and diffuse membranous and/or cytoplasmic CD-56 staining. 28/36 (77.78%) cases of UTPTC and 24/25(96%) cases of FVPTC were seen to have absent CD-56 expression. The difference in CD-56 expression between FVPTC (1/25,4%) and FA & its variants (20/24,83.33%) and FCa (6/7,85.71%) was highly statistically significant (p-value <0.01). Clinico-pathological parameters such as male: female ratio, mean age, mean tumour size, tumour staging, histologic grading [Table/Fig-5] of CD-56 positive and negative cases were studied. The mean age at diagnosis of CD-56 positive PTC cases was 48.11y±8.81 and that of CD-56 negative PTC cases was 38y±3.698. The difference between the mean ages was highly statistically significant (p<0.01). The mean tumour size of CD-56 positive PTC cases was 5.97cm±8.81 and that of C D56 negative PTC cases was 4.83cm±3.698, however the difference between the mean tumour sizes was statistically insignificant (p=0.507). The sensitivity, specificity of CD-56 expression in diagnosing PTC was 85.86%, and 85.25% respectively. The positive and negative predictive values were 90.43% and 78.79%, respectively.
p53 overexpression was seen in 58/68 (85.29%) malignant STN cases [Table/Fig-4]. In the non-malignant group, 27/92 cases (29.35%) demonstrated p53 positivity. The difference in p53 expression between the malignant and non-malignant STN cases was found to be highly statistically significant (χ2=46.924, p<0.001). Also on comparing PTC cases (52/61, 85.25%) with non-neoplastic lesions (12/68, 17.65%) for p53 overexpression, the difference was found to be highly statistically significant (χ2=56.105, p<0.001). However, the difference in p53 expression between FA (15/24, 62.5%) versus FCa (6/7, 85.71%) [Table/Fig-2] and between FA (15/24,62.5%) versus FVPTC (21/25,84%) was not observed to show any statistical significance (p=0.379). The sensitivity and specificity of p53 IHC marker in differentiations malignant and non-malignant lesions/tumours was 85.29% and 70.65% respectively. Its positive and negative predictive values were 68.24% and 86.67% respectively. The diagnostic accuracy was 76.88%. Clinico-pathological parameters such as age, tumour size, extrathyroidal extension, lymphovascular invasion, lymph node metastasis [Table/Fig-6] were studied. The mean age at diagnosis of p53 positive PTC cases was 39.5y±8.81 and that of p53 negative PTC cases was 43.5y±3.698. However the difference between the mean ages was statistically insignificant. (p=0.164). The mean tumour size of p53 positive PTC cases was 5.18cm±8.81 and that of p53 negative PTC cases was 5.21cm±3.698, however the difference between the mean tumour sizes was statistically insignificant (p=0.992).
Comparison of p53 positive overexpression in malignant cases versus p53 negative malignant cases.
| Parameters | p53positivecases(n = 58) | p53negativecases(n = 10) | p-value |
|---|
| Female | 45 | 8 | |
| Male | 13 | 2 | |
| Mean age atdiagnosis (yrs) | 39.5 | 43.5 | p=0.164 |
| Mean tumoursize at diagnosis(cms) | 5.18 | 5.21 | p=0.992 |
| Lymphnodemetastasis | Positive | 11(18.97%) | 01(10%) | p=0.812 |
| Negative | 47(81.03%) | 09(90%) |
| Extrathyroidalextension | Positive | 01(1.72%) | 0 | p=1.00 |
| Negative | 57(98.28%) | 10 (100%) |
| Lymphovascularinvasion | Positive | 06(10.34%) | 02(20%) | p=0.334 |
| Negative | 52(89.66%) | 08(80%) |
| TNMstaging | Stage I | 38(65.52%) | 08(80%) | χ2=1.046;p=0.593 |
| Stage II | 03(5.17%) | - |
| Stage III | 17(29.31%) | 02(20%) |
| Stage IV | 0 | 0 |
| Grading | Grade I | 50 (86.21%) | 10(100%) | p=0.593 |
| Grade II | 08(13.79%) | 0 |
The mean proliferative Ki-67 index is mentioned in [Table/Fig-7]. The statistical difference in mean Ki-67LI was found to be significant between PTC versus FA (p<0.05), FA versus FCa(p<0.05) and FVPTC(3.34±2.04) versus FA (p<0.05) [Table/Fig-2f] The difference in mean Ki-67LI was also found to be highly statistically significant (p-value <0.01) between non-neoplastic lesions and PTC. The mean Ki-67 LI in cases of malignant tumour (68 cases) was 3.47±2.33 and non-malignant lesions/tumour was 1.37±0.94, the difference being highly statistically significant (p<0.01). The difference in mean of Ki-67 LI in distinguishing non neoplastic, FA, FCa and PTC was statistically significant. (F=39.57, p<0.01) However, the exact cut off value to determine Ki-67 positivity or negativity could not be calculated as the sample size required for this purpose was not adequate in this study.
Ki-67 labeling index in Non-neoplastic and Neoplastic STN lesions (n = 160).
| Diagnosis | No of cases(n = 160) | Ki-67 Labeling index |
|---|
| Mean±SD | Range |
|---|
| PTC | 61 | 3.45±2.40 | 0.5 – 15 |
| FCa | 07 | 3.71±1.49 | 2 - 7 |
| FA | 24 | 2.23±1.05 | 0.5 – 4 |
| Non-neoplastic | 68 | 1.07±0.66 | 0.5 – 3 |
F=39.57, p-value < 0 .01 (ANOVA)
STN-solitary thyroid nodule, PTC- papillary thyroid carcinoma, FCa-follicular carcinoma, FA-follicular adenoma.
Discussion
Several authors have attempted to distinguish benign STN from malignant ones and papillary carcinomas from follicular tumours of the thyroid and non-neoplastic lesions on the basis of IHC evaluation using various antibodies to cytokeratins and analysing its differential expression in normal and neoplastic follicular cells. Although certain studies have concluded that these antibodies are beneficial in the distinction, others have concluded equivocal results.
CK-19 was found to be a sensitive (83.61%) and a highly specific marker (100%) for the diagnosis of PTC in the present study considering 4+ as the cut off value. In various studies it was reported that CK-19 is a sensitive marker for PTC. However, it was found to be less specific [12,17]. We found that 83.61% of the PTC cases which included 77.78% of UTPTC and 92% of FVPTC cases showed diffuse CK-19 positivity. The rest of the 10 cases (16.39%) comprising of 8 UTPTC and 2 FVPTC showed focal staining for CK-19 and were considered negative. The presence of strong and diffuse CK-19 staining in a thyroid malignancy with a follicular growth pattern should raise the suspicion of FVPTC, and the lesion should be assessed carefully for the presence of the nuclear and other features of PTC. Occasionally, the CK-19 staining of PTC (including FVPTC) can be patchy rather than diffuse, as demonstrated by our study and by other researchers in this field [11]. Therefore, less than diffuse staining for CK-19 does not rule out a diagnosis of FVPTC if the diffuse nuclear changes typical of this type are present.
An 84.2% of UTPTC and 62.5% of FVPTC showed CK-19 positivity using 4+ as cut off value in one study [11]. A 93% of FVPTC and 100% of UTPTC showed diffuse CK-19 positivity in another study [10]. Cheung CC et al., reported diffuse CK-19 staining in 80% (43/54) of UTPTC and 57% (48/84) of FVPTC. They observed that 17% (6/35) of FA showed focal staining with CK-19 and 3% (1/35) showed diffuse CK-19 staining [17]. Its reactivity in follicular lesions/tumour may limit its utility as a diagnostic marker [5]. However, none of the non-neoplastic and benign tumours showed diffuse CK-19 positivity in the present study. Thus CK-19 was found to be a useful marker for differentiating PTC from papillary hyperplasia seen in non-neoplastic lesions. No difference was found in CK-19 expression between UTPTC and FVPTC as stated in literature [10,11]. In this study, we analysed the correlation of clinico-pathological variables with CK-19 expression and did not find it to be useful in determining the severity of PTC in patients. We could not find a reference study assessing the correlation of CK-19 with clinico-pathological parameters.
Membranous CD-56 expression is confirmed in thyroid follicular cells [13]. It is documented that modifications of CD-56 expression in PTC causes down-regulation of vascular endothelial growth factor D which stimulate lymphangiogenesis [18].
In this study, CD-56 was found to be a sensitive (85.86%) and specific (82.25%) marker in differentiating PTC (especially FVPTC) and follicular lesions/neoplasms similar to other studies in literature [13]. A study has demonstrated 95.8% (70/73) positive and 98.63% (72/73) negative CD-56 expression for non-papillary thyroid lesions and PTC cases respectively [13]. In another study, there was 100% CD-56 expression in thyroid follicular lesions. The tumour centers of PTC cases were devoid of CD-56 staining [12]. CD-56 gene was expressed in 93% of benign and 5% of PTC cases respectively giving sensitivity and specificity of 94.8% and 92.3% respectively [19].
A study has demonstrated 89.4% (42/47) CD-56 positivity and 82.8% (24/29) CD-56 negativity in follicular patterned thyroid nodules and PTC cases respectively. The sensitivity and specificity of CD-56 in differentiating FVPCs and other follicular nodules as per their study was 81.3% and 89.4% [20]. However, Etem H et al., found no statistically significant difference between FVPTC’s and follicular tumours for CD-56 expression [21]. In the present series, we analysed the correlation of clinico-pathological variables of PTC cases with CD-56 expression and found that PTC cases showing negative CD-56 expression had an earlier age of presentation and majority were of histologic grade 1. Other factors did not have usefulness in assessing the disease severity of PTC. We could not find a similar study in literature.
The concept that p53 is implicated in advanced cancer progression is contradicted by a series of evidences pointing that it plays an important role in the early stages of thyroid cancer [22]. Increased p53 protein levels were observed by IHC in anaplastic, poorly differentiated and well-differentiated thyroid cancers in the absence of p53 mutation [23]. It has been currently demonstrated that the wild-type protein overexpression resulting from unidentified factors causes a protective mechanism in tumours. A total of 11-59% of PTC cases overexpress p53 protein [24].
The sensitivity and specificity of p53 in differentiating malignant tumours from other non-malignant lesions/tumours was 85.29% and 70.65% respectively in the present study. Several studies recently showed that p53 was significantly increased in PTC patients when compared with benign thyroid disorders. They have reported that the detection of p53 protein was a significant and independent prognostic indicator in differentiated thyroid carcinoma [15,16,23,24].
A study reported 77.4% (127/164) of well differentiated PTC and 26.2% (11/42) FCa cases were p53 positive. 25.7% (27/105) of benign lesions (mainly FA) presented p53 positivity. A study done by Stan V et al., showed 7.7% (2/26) PTC and 20% (2/10) FCa cases presented focal, mild/moderate p53 expression and it was absent in FA and nodular hyperplasia, the results being statistically insignificant. p53 immunostaining in their study did not prove to be useful in differentiating PTC from nodular hyperplasia and also FA from FCa [22]. In addition, Marcello MA et al., reported that p53 was more often seen staining smaller tumours (<2 cm) than tumours larger than 4 cm (p < 0.01). p53 was more frequent in solitary nodules than in multifocal tumours (p = 0.0286) and tended to appear more frequently in encapsulated tumours than in those without capsule, although the statistical comparison showed a marginal p-value (p = 0.0762) [23]. Lee Y et al., showed that p53 expression was significantly higher in PTC tumours found in male patients. However, they found that p53 expression was not significantly associated with extrathyroidal extension and lymph node metastasis [16]. Shin M et al., showed p53 protein overexpression in 47.3% of the PTC cases. The diagnostic sensitivity and specificity were 85.0% and 72.7%, respectively. However, no significant correlation between p53 protein overexpression and clinicopathologic features (age, size, lymph node metastasis, extrathyroidal extension and vascular invasion) was noted. These outcomes were consistent with findings of our study [24].
In the present study, there was statistically significant difference in Ki-67 LI between malignant and non-malignant thyroid tumours/lesions, follicular adenoma and PTC, FCa, FVPTC and non-neoplastic lesions and PTC as seen in literature [25–27]. Ki-67 LI was observed to be an independent prognostic factor for disease-free survival, and that patients with high Ki-67 LI values had a significantly worse disease-specific survival than in patients with low values in a study done by Miyauchi A et al., [27].
Limitation
The limitations of the present study was the small sample size consisting of a limited number of cases of FA and FCa. Therefore, the cut off value for Ki-67 for distinguishing benign from malignant follicular neoplasms could not be determined.
Conclusion
CK-19 was found to be a sensitive (83.61%) and a highly specific positive marker (100%) for the diagnosis of PTC in the present study considering 4+ as the cut off value. CD-56 was found to be sensitive (85.86%) and specific (82.25%) negative marker in differentiating PTC from follicular neoplasms. The sensitivity and specificity of p53 marker in separating malignant and benign tumours was 85.29%, and 70.65% respectively. The difference in mean Ki-67LI in cases of malignant and benign tumour was highly statistically significant (p<0.01).
Thus, a panel of four IHC markers (CK-19, CD-56, p53, Ki-67) may be used for differentiating doubtful benign STN cases from malignant ones and also for definitive diagnosis of PTC along with histopathological examination.
STN-solitary thyroid nodule, FCa-follicular carcinoma, UTPTC-usual type papillary thyroid carcinoma FVPTC-follicular variant of papillary thyroid carcinoma.
FCa-follicular carcinoma, UTPTC-usual type papillary thyroid carcinoma FVPTC-follicular variant of papillary thyroid carcinoma, PTC-papillary thyroid carcinoma
STN-Solitary thyroid nodule, IHC-immunohistochemistry, FCa-follicular carcinoma, PTC- papillary thyroid carcinoma, UTPTC-usual type papillary thyroid carcinoma FVPTC-follicular variant of papillary thyroid carcinoma.
PTC- papillary thyroid carcinoma.
F=39.57, p-value < 0 .01 (ANOVA)
STN-solitary thyroid nodule, PTC- papillary thyroid carcinoma, FCa-follicular carcinoma, FA-follicular adenoma.