Papulonodular lesions of skin occur due to various neoplastic and non-neoplastic conditions. The commonest diagnostic method used is skin biopsy. However, Fine Needle Aspiration Cytology (FNAC) of skin lesion is a relatively new comer and has not achieved much popularity as the cytological assessment in lesions at other sites [1]. Skin cytology is without doubt a rapid easy technique in experienced hands and it is possible to provide valuable information in certain conditions. The procedure is sometimes preferable to biopsy from areas such as face to avoid or minimize scarring [1].
FNAC is useful in differentiating benign from malignant tumour and also in diagnosing primary skin tumour. Some of the lesions that can be diagnosed on FNAC include epidermal inclusion cyst, trichilemmal cyst, Basal Cell Carcinoma (BCC), Squamous Cell Carcinoma (SCC), melanoma, sebaceous carcinoma, merkel cell tumour, pilomatrixoma, granular cell tumour, Kaposi sarcoma and various skin adnexal tumours [2]. Some of the subcutaneous nodules diagnosed by FNAC showed metastases from cervix, lung, breast, prostate, ovary, liver, kidney and gall bladder [3].
FNAC is useful in diagnosis of many infective conditions presenting as nodular swelling of skin. It is useful in diagnosing parasitic infections like cysticercosis [4], fungal infection like aspergillosis [5], Erythema Nodosum Leprosum (ENL) [6], lepromatous leprosy and Molluscum contagiosum [7].
Hence, this study was undertaken to study the FNAC findings in various papulonodular lesions and to correlate with histopathology wherever possible.
Materials and Methods
Fifty cases of clinically diagnosed papulonodular lesions, who attended the Departments of Dermatology and Surgery in a Medical College attached tertiary care center, Karnataka for a period of two years from 2012-2014 were studied. Ethical clearance was obtained by the University Ethical Committee. A brief history was taken and the patients were explained of the simple procedure of Fine Needle Aspiration (FNA) and written consent was taken. After examining briefly the nature of lesions, FNAC was done under aseptic precautions. Two to three passes were adequate to obtain a satisfactory sample. Smears were stained with routine stains like May Grunwald Geimsa (MGG), Haematoxylin and Eosin (H&E) and Papanicolaou (PAP). Special stains like Periodic Acid Schiff (PAS), Gomori Methenamine Silver (GMS), Ziehl-Neelsen and Fite-Faracco was done wherever necessary.
Biopsy was done under local anaesthesia and the sample was placed in gauze moistened with saline solution in a closed petridish and fixed in 10% formalin. After routine processing and paraffin embedding, H&E sections were studied. Special stains were used wherever necessary. Routine investigations like haemoglobin percentage, total count, differential count, peripheral blood smear, chest X-ray and ultrasound abdomen were advised whenever necessary.
Results
The various papulonodular lesions of skin ranged from inflammatory to neoplastic and metastatic deposit [Table/Fig-1]. It was observed that inflammatory lesions and epidermal cysts occur in all age groups with inflammatory lesions being common in the age group of 21-30 years and epidermal cysts between 31-40 years with a mean age of incidence between 28 and 38 years. Secondaries were seen in the age group of 31-70 years with a mean age incidence of 58 years [Table/Fig-2,3]. In the present study more number of male patients presented with papulonodular lesions of skin with a male to female ratio of 1.2:1 [Table/Fig-4].
Various papulonodular lesions of skin.
| Frequency | Percentage (%) |
|---|
| Epidermal Cyst | 13 | 26 |
| Inflammatory Lesions |
| Bacterial |
| Acute inflammatory lesion | 05 | 10 |
| Chronic non-specific | 03 | 06 |
| Leprosy | 05 | 10 |
| Tuberculosis | 02 | 04 |
| Viral |
| Molluscum contagiosum | 01 | 02 |
| Actinomycosis | 01 | 02 |
| Foreign body granuloma | 02 | 04 |
| Primary Neoplastic Lesions |
| Lipoma | 03 | 06 |
| Benign adnexal tumour | 02 | 04 |
| Pilomatrixoma | 01 | 02 |
| Basal cell carcinoma | 01 | 02 |
| Metastatic Deposit (Secondaries) | 05 | 10 |
| Non-Specific | 06 | 12 |
| Total | 50 | 100 |
Age distribution of papulonodular lesions.
| Age group (in years) | Papulonodular lesions | Total |
|---|
| Epidermal cyst | Inflammatory lesions | Primary neoplastic lesions | Secondaries | Non-specific |
|---|
| 0-10 | 3 | 1 | 1 | | | 5 |
| 23.1% | 5.3% | 14.3% | | | 10.0% |
| 11-20 | 3 | 1 | 1 | | 1 | 6 |
| 23.1% | 5.3% | 14.3% | | 16.7% | 12.0% |
| 21-30 | 2 | 6 | 3 | | 1 | 12 |
| 15.4% | 31.6% | 42.9% | | 16.7% | 24.0% |
| 31-40 | 3 | 3 | | 1 | 1 | 8 |
| 23.1% | 15.8% | | 20.0% | 16.7% | 16.0% |
| 41-50 | 1 | 4 | 2 | 1 | 1 | 9 |
| 7.7% | 21.1% | 28.6% | 20.0% | 16.7% | 18.0% |
| 51-60 | | 3 | | | 2 | 5 |
| 15.8% | | | 33.3% | 10.0% |
| 61-70 | | 1 | | 2 | | 3 |
| 5.3% | | 40.0% | | 6.0% |
| 71 & above | 1 | | | 1 | | 2 |
| 7.7% | | | 20.0% | | 4.0% |
| Total | 13 | 19 | 7 | 5 | 6 | 50 |
| 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% |
Standard deviation (SD) and mean age incidence of various lesions.
| No. | Mean | SD | Minimum | Maximum |
|---|
| Epidermal cyst | 13 | 28.00 | 19.43 | 1 | 75 |
| Inflammatory | 19 | 37.37 | 15.79 | 4 | 68 |
| Primary neoplastic lesions of skin | 7 | 28.14 | 15.55 | 3 | 50 |
| Secondaries | 5 | 57.20 | 17.43 | 31 | 72 |
| Non-specific | 6 | 41.67 | 15.91 | 20 | 59 |
| Total | 50 | 36.14 | 18.46 | 1 | 75 |
Sex distribution of all lesions.
| Disease | Males | Females | Total number of cases |
|---|
| No. of cases | Percentage | No. of cases | Percentage |
|---|
| Primary neoplastic lesions | 03 | 92.8 | 04 | 57.2% | 07 |
| Secondaries | 05 | 100 | 0 | 0 | 05 |
| Epidermal cyst | 08 | 61.54 | 05 | 38.46 | 13 |
| Inflammatory lesions | 07 | 36.8 | 12 | 63.2 | 19 |
| Non specific | 04 | 66.6 | 02 | 33.4 | 06 |
[Table/Fig-5] shows that diagnostic accuracy of FNAC compared to histopathology was available in 46% of the cases. 100% accuracy was seen in cases of epidermal cysts, leprosy, tuberculosis and benign adnexal tumours. A total of 50% accuracy was seen in pilomatrixoma and BCC. Sensitivity for epidermal cyst and inflammatory lesions was 100%. In case of adnexal tumours sensitivity and specificity was 66.7% and 50% respectively.
Correlation between FNAC and histopathology based on gender.
| Lesions | FNAC | Total | HPE | Accuracy % |
|---|
| Malen (%) | Femalen (%) | Correlated | Not correlated |
|---|
| Epidermal cyst | 4 (44.5%) | 3 (21.5%) | 7 | 7 | 0 | 100 |
| Inflammatory |
| Acute inflammatory | 0 | 2 (14.2%) | 2 | 2 | 0 | 100 |
| Chronic non-specific inflammatory lesion | 0 | 2 (14.2%) | 2 | 2 | 0 | 100 |
| Leprosy | 0 | 3(21.5%) | 3 | 3 | 0 | 100 |
| Tuberculosis | 1 (11.1%) | 0 | 1 | 1 | 0 | 100 |
| Actinomycosis | 1 (11.1%) | 0 | 1 | 1 | 0 | 100 |
| Primary neoplastic lesion |
| Adnexal tumour | 1 (11.1%) | 0 | 1 | 1 | 0 | 100 |
| Pilomatrixoma | 0 | 1 (7.1%) | 1 | 1 | 0 | 50 |
| Basal cell carcinoma | 0 | 1 (7.1%) | 1 | 0 | 1 | 50 |
| Non specific | 2 (22.2%) | 2 (14.2%) | 4 | 1 | 03 | 25 |
| Total | 9 (100%) | 14 (100%) | 23 (100% | 19 | 04 | 83% |
Discussion
The present study was an attempt to assess the diagnostic accuracy of FNAC on papulonodular lesions of skin. In recent years FNAC has replaced biopsy as a diagnostic procedure and is preferred as a pre-operative non-invasive procedure. Epidermal cyst was the most common lesion we encountered [Table/Fig-6a] and this correlates well with the study by Butler ED et al., who described that epidermal inclusion cysts were commonly seen in subcutaneous tissue, pre-sacral area and hand, and were frequently subjected to FNA mainly to confirm the clinical diagnosis [8]. Out of 13 cases, 7 cases (53.8%) underwent histopathological examination and all of them correlated [Table/Fig-6b]. FNAC features correlated well with the studies done by Eimani et al., and Shet TM et al [9,10]. The diagnostic accuracy of epidermal cyst FNA in the present study was 100%.
a,b) FNA smear (MGG, x400) and histopathology of Epidermal cyst (H&E, x400); c,d): FNA smears displaying features of lepromatous leprosy (H&E, x400, Fite-Faracco, x1000).
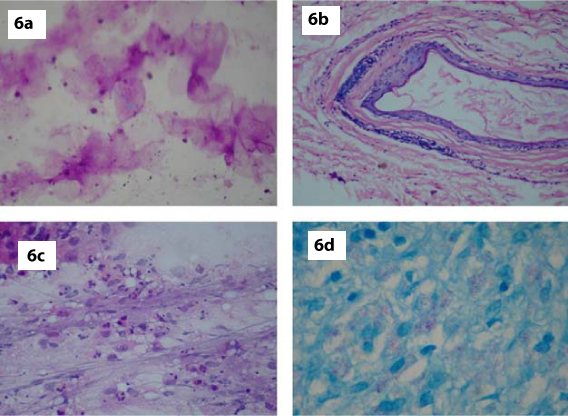
Our study showed 5 cases of Leprosy [Table/Fig-1] with female preponderance comprising of 3 females and 2 males (M:F = 1:1.5). Extremities were the most common site and the maximum number of cases was seen in the age group 21-40 years. This correlated well with the study by Singh N et al., who described that Hansen’s disease is a slowly progressive infection affecting the peripheral nerves and extremities [11]. Out of 5 cases, 3 cases (60%) underwent histopathological examination and all of them were diagnosed as lepromatous leprosy [Table/Fig-6c,d]. This correlated well with the studies done by Anshu and Singh N et al., [6,11].
Singh N and Bhatia et al., in their study of 30 cases of leprosy, noted that the majority of cases of lepromatous leprosy showed increased cellularity, numerous foamy macrophages, and few lymphocytes with all the cases positive for Acid Fast Bacilli (AFB). Tuberculoid leprosy showed cohesive epithelioid granulomas and negative for AFB [Table/Fig-7a]. Thus, the findings of present study correlated with the study by Anshu et al., [6].
a) FNA smear showing features of tuberculoid leprosy (MGG, x400); b) Section showing skin with numerous Molluscum bodies-Molluscum contagiosum (H&E,x100); c,d) FNA smear and corresponding histopathology displaying features of Lupus vulgaris (MGG, x400 & H&E, x100).
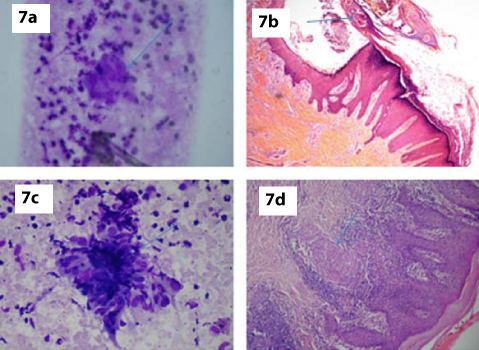
We reported one case of Molluscum contagiosum [Table/Fig-1] in a four-year-old patient presenting with multiple umbilicated nodular swelling over the face. FNAC smears showed neutrophils and pathognomonic Molluscum bodies. Jain S and Das D et al., described similar FNAC findings in a four-month-old female presenting with papule over the back and chest wall [7]. Inclusion bodies are seen in three groups of virus causing skin lesions, Herpes virus, Papilloma virus and Pox viral groups. The first and second group of viruses are characterized by presence of intra-nuclear inclusions, while the third group is characterized by intra-cytoplasmic and intra-nuclear inclusions. The inclusions in Molluscum contagiosum are classically intra-cytoplasmic [Table/Fig-7b] [7].
Out of 50 cases, 2 cases were diagnosed as tuberculosis of skin (Lupus vulgaris) and one case was confirmed by histopathological examination [Table/Fig-7c,d]. Bhatia et al., noted similar findings on FNAC. He also noted that cytological findings in tuberculoid leprosy, sarcoidosis, and in lupus vulgaris were similar [12]. Hence, other clinical features and laboratory investigations may be required for differentiating above conditions.
Two cases of foreign body granulomas were reported in our study [Table/Fig-1]. Bhatia A et al., in their study on granulomatous inflammation of the skin stated that foreign body granulomas were characterized by multinucleated giant cell, acute inflammation and demonstrable foreign bodies [Table/Fig-8a]. Thus, the findings of the present study correlated with their study [12]. Silica, talc, exogenous lipid induce granulomatous reactions within the dermis. Residual particles of talc, silica and lipids are demonstrable in tissue by routine or polarising microscopy. Arthropod bite may on occasion cause inflammatory and granulomatous reactions [13].
a) FNA smear showing features of foreign body granuloma (MGG, x100); b) FNA smear showing features of lipoma (H&E, x200); c) Histopathology showing actinomycotic colony(H&E,x100); d) Histopathology showing features of pilomatrixoma (H&E, x200).
MGG-May Grunwald Geimsa; FNA- Fine Needle Aspiration
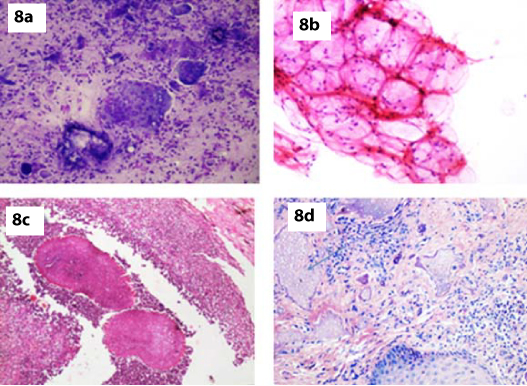
We reported three cases of lipoma which on cytology showed adipose tissue which was indistinguishable from normal fibroadipose tissue. The fat cells were univacuolated with nuclei pushed to the periphery [Table/Fig-8b]. Histopathology confirmed the cytology diagnosis in all three cases.
In the present study, there was one case of actinomycosis which showed acute inflammatory cell infiltrate and epithelioid cell granulomas in a proteinaceous background. Gram stain done showed gram positive filamentous structures. Bhatia A et al. noted similar findings on FNA cytology. Demonstration of gram positive filaments in smears and positive cultures were confirmatory [12]. Actinomycosis was confirmed subsequently on histopathological examination [Table/Fig-8c].
Our study reported one case of pilomatrixoma in a 40-year-old male. Pilomatrixoma is a nodular subepidermal benign tumour arising from the hair matrix. It characteristically present as a solitary, firm, slow growing subepidermal nodule [13]. According to the study done by Stanislaw Woyke et al., the most common location of the tumour is head, neck and upper extremities. The tumour affects male and female at any age but mostly children and adolescents [14]. Cytological examination of smear in present study showed ghost cells, basaloid cells and numerous acute inflammatory cells. Subsequent histopathological examination confirmed the diagnosis of pilomatrixoma [Table/Fig-8d]. The cytological features correlated with the study done by Henry KA et al., and Ma KF et al., [15,16].
One case of pilomatrixoma was misdiagnosed as basal cell carcinoma in our study. Cytology smears showed basaloid cells and numerous acute inflammatory cell infiltrates. On histopathology it was diagnosed as pilomatrixoma. Ma KF et al., stated that, there is a risk of making a false positive cytological diagnosis in this tumour, particularly if basaloid cells dominate the smears and the characteristic ghost cells are not present [16].
BCC was an important diagnostic consideration in our case because of the predominance of basaloid cells. BCC is characterized cytologically by cohesive cellular fragments with sharp borders and peripheral palisading. The centrally located cells were present in a disorderly arrangement with nuclear crowding and overlapping. Histopathology confirmed the diagnosis [Table/Fig-9a]. Shadow cells and foreign body reaction were absent. The presence of ghost cell in one aspirate helped us to establish the correct diagnosis of pilomatrixoma without difficulty.
a) Histopathology showing features of Basal cell carcinoma (H&E, x100); b,c) FNA smear and corresponding histopathology showing features of Cylindroma (H&E, x100, H&E, x100); d) FNA smear displaying features of metastatic adenocarcinoma deposit (MGG,x400).
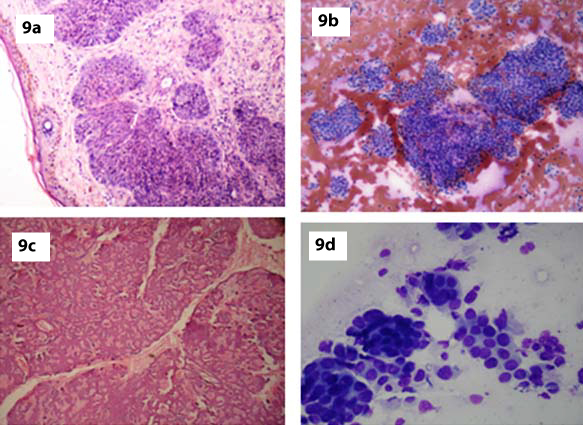
The clinical history, absence of atypia, mitotic figures and presence of ghost cell along with cohesiveness of clusters help to arrive at a definitive diagnosis and avoid a false positive diagnosis of malignancy [17]. The diagnostic accuracy of pilomatrixoma was 50% in the present study.
The available literature on the cytological findings of tumours of the skin adnexae is scanty. The lesions cytologically well recognized include pilomatrixoma and Merkel cell tumour. In the present study two cases of benign adnexal tumours were reported and because of classical location of one tumour on the scalp it was reported as cylindroma and the other case occurred on the face [Table/Fig-5]. In both the cases smear showed sheets and clusters of scattered small cells with scanty cytoplasm and large round nuclei [Table/Fig-9b]. One of the case was confirmed by histopathological examination as cylindroma [Table/Fig-9c].
There is a wide variety of skin adnexal tumours and making a diagnosis on histologic section requires experience in this field. Recognition of the nature of adnexal tumour whether it is benign or malignant will guide the surgeon to outline treatment and follow up. Shet T et al., and Rege J stated that the exact subtyping of the skin adnexal tumour on FNAC may not always be essential [10,18].
Cutaneous or subcutaneous nodules from carcinomas of internal organs are relatively rare. The occurrence of such metastasis with the primary neoplasm alters the clinical stage of the disease and hence, management [19]. In the present study, 5 cases of cutaneous metastasis were reported. All of them were male patients with age ranging from 31 to 75 years [Table/Fig-3]. In the study done by Agarwal J and Gupta JK, the age of the patients ranged from 15 to 70 years (Mean 57 years) with male preponderance (55.2%) as compared to females (44.1%) [3]. In the present study, there were 3 (60%) cases of metastatic adenocarcinoma and 2 cases of poorly differentiated carcinoma. Primary lesions were identified in 4 cases by using radiological and endoscopy findings (1 from stomach, 2 from lung, 1 from adrenal gland).
Agarwal J and Gupta JK studied 68 cases of metastatic cutaneous nodules. The commonest site of primary tumour was lung in males and cervix, breast followed by ovary in females [3]. Brownstein et al., described that the most common source of metastatic carcinoma to the skin was from lung (25%), large bowel, melanoma, kidney and epidermoid carcinoma of the oral cavity in males and breast (69%) followed by lung, malignant melanoma, kidney and ovary in females [19]. Three cases of metastatic adenocarcinoma were seen which showed tumour cells in acinar pattern and sheets having vesicular nuclei, prominent nucleoli and vacuolated cytoplasm.
In our study, majority of the cases were diagnosed as adenocarcinoma deposits [Table/Fig-9d]. This correlates with the study by Srinivasan R et al., and Reye CV et al., who noted 29 and 27 cases of adenocarcinoma out of total number of 38 and 58 cases [20,21].
Two (40%) cases of poorly differentiated carcinoma were seen which showed highly cellular smear with cells having high N:C ratio and tumour giant cells. Primary right adrenal lesion was identified in one case. Gupta RK and Naran S described that immunostaining studies may be useful procedure for defining the site of primary [22]. In our study of metastatic cutaneous deposit, the diagnostic accuracy was 100% and similar results were reported by Cesar VR et al., and Shet T et al., [21,18].
Thus, aspiration cytology along with radiological studies proved very useful in classifying the nature of cutaneous nodules, eliminating the need of a surgical biopsy and histopathological examination and suggesting the possible site of unknown primary. It can also detect or exclude relapse of previously treated malignancies. FNA can play an important role in the diagnosis and therapeutic planning of these tumours [3]. Hence, FNAC is an useful procedure for the detection and confirmation of metastatic deposits from internal carcinoma in palpable cutaneous and subcutaneous nodules.
Limitation
The study had a few limitations like histopathology was not available for all the cases for correlation. Also, studies involving a bigger sample size will be more helpful to assess the significance of this correlation.
Conclusion
Skin FNAC is valuable in solving problems of clinical papulonodular lesions. It will provide definite diagnosis in majority of cases especially lepromatous leprosy, lupus vulgaris and other bacterial infection of skin to enable immediate treatment. FNAC is a technique that is cost effective, of high diagnostic accuracy and results in considerable resource saving. Thus, FNAC is a quick, safe and a less traumatic procedure to diagnose nodular lesions of skin.