Role of Altered Venous Blood Lactate and HbA1c in Women with Gestational Diabetes Mellitus
C S Nagalakshmi1, N U Santhosh2, N Krishnamurthy3, Chethana Chethan4, M K Shilpashree5
1 Associate Professor, Department of Biochemistry, Akash Institute of Medical Sciences and Research Centre, Devanahalli, Bangalore, Karnataka, India.
2 Consultant Neurosurgeon (Endovascular), Department of Neurosurgery, Aster CMI Hospital, Sahakar Nagar, Bangalore, Karnataka, India.
3 Professor and Head, Department of Biochemistry, BGS Global Institute of Medical Sciences, Kengeri, Bangalore, Karnataka, India.
4 Associate Professor, Department of Biochemistry, BGS Global Institute of Medical Sciences, Kengeri, Bangalore, Karnataka, India.
5 Assistant Professor, Department of Biochemistry, BGS Global Institute of Medical Sciences, Kengeri, Bangalore, Karnataka, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. C S Nagalakshmi, Associate Professor, Department of Biochemistry, Akash Institute of Medical Sciences and Research Centre, Prasannahalli Road, Devanahalli, Bangalore-562110, Karnataka, India.
E-mail: nagu.smile@gmail.com
Introduction
Being a mirror image of metabolic syndrome, Gestational Diabetes Mellitus (GDM) is associated with significant maternal and fetal morbidity. Increased blood lactate concentration and alterations of substrate utilization are partly involved in development of insulin resistance in GDM. Fetuses born to such mothers have shown low umbilical vein oxygen saturation and low oxygen content and increased lactate concentrations. These changes may certainly reflect enhanced fetal metabolism as a result of hyperglycaemia and hyperinsulinemia and therefore, these fetuses deserve intense surveillance at term and during delivery. Ideally, HbA1c should be maintained below 5% during their first trimesters and below 6% during third trimester. We planned to investigate GDM women for their HbA1c levels too.
Aim
To know if there is any alteration in blood lactate and/or HbA1c levels and to know if there is any correlation between these two parameters in GDM pregnancies, in comparison with the previous studies which measured lactate in cord blood and placental vessels of GDM women.
Materials and Methods
It was a hospital based prospective study on 40 women with gestational diabetes and 40 age-matched normal pregnant women. We analysed the biochemical and metabolic mileau in these women by estimating venous blood lactate and HbA1c levels. We paid special attention to follow them up regarding maternal complications if any and perinatal outcomes. The independent samples t-test and Pearson’s correlation test were applied.
Results
GDM mothers showed significantly higher lactate and HbA1c levels than normal pregnant women, both with p<0.001. Blood pressure and fetal birth weight were also significantly higher in GDM group than Normal Pregnant (NP) group, both with p-values of <0.001. Further, this increased lactate levels showed significant positive correlation with HbA1c, blood pressure and fetal birth weight.
Conclusion
Maternal blood lactate and HbA1c levels have a significant role to play in determining the metabolic mileau of both mother and fetus and thus, their obstetric and general health outcomes.
Lactate concentration, Metabolic syndrome, Maternal and fetal morbidity
Introduction
Around 2-5% of pregnancies are reported to develop Gestational Diabetes Mellitus (GDM) annually. Being a mirror image of metabolic syndrome, it is associated with significant maternal and fetal morbidity. Further, it is comparable to type 2 Diabetes Mellitus (DM) in its causation, presenting features, biochemical alterations and consequences [1,2].
Various regulatory hormones and growth factors have been implicated in the pathogenesis of type 2 DM and GDM and one such novel molecule considered for study these days is lactate, an intermediate in carbohydrate metabolism. It is derived pre-dominantly from white skeletal muscle, brain, skin, renal medulla and erythrocytes and is metabolized by liver and kidneys [1]. Nearly, 65% of total basal lactate production is used by liver for gluconeogenesis. Its extrahepatic removal is by oxidation in red skeletal muscle and renal cortex. A moderate increase in lactate production results in increased hepatic clearance, but it becomes saturated at concentrations >2mmol/L.
Normal individuals and type 2 DM patients have shown a significant negative correlation between blood lactate and blood glucose levels, whereas, a significant positive correlation has been established between blood lactate and IRCP (Immuno Reactive C-Peptide) levels [3]. Increased blood lactate concentration and alterations of substrate utilization are partly involved in development of insulin resistance in such subjects, via greater carbohydrate oxidation [4].
At birth, fetuses born to GDM mothers have shown significantly lower umbilical vein oxygen saturation and low oxygen content and increased lactate concentrations. These changes may certainly reflect enhanced fetal metabolism as a result of hyperglycaemia and hyperinsulinemia and therefore these fetuses deserve intense surveillance at term and during delivery [5].
Further, it has been shown through studies that, for prevention of congenital malformations and macrosomia in fetuses of GDM mothers, HbA1c should be maintained below 5% during their first trimesters and below 6% during third trimester [6].
Although, studies have been done showing altered lactate levels in placental vessels of gestational diabetic women, variations in lactate levels in venous blood of GDM women has not been elucidated. Considering the importance attached to lactate and HbA1c in diabetes, we planned a study to know if there is any alteration in blood lactate and/or HbA1c levels and to know if there is any correlation between these two parameters in GDM pregnancies, in comparison with the previous studies which measured lactate in cord blood and placental vessels of women affected by GDM.
Materials and Methods
This was a prospective study conducted from January to September 2010. It involved 80 pregnant women between the age groups 18-37 years: forty women with Gestational Diabetes (GDM group) and 40 age-matched Normal Pregnant women (NP group). Subjects were recruited from the Department of Obstetrics and Gynaecology from a tertiary care hospital in Karnataka after obtaining institutional Ethical Clearance. Three ml of venous blood was collected from a stasis free vein with all aseptic precautions from both the groups between their gestational ages 24-28 weeks. Immediately after collecting, blood was stored in an ice bath and the plasma was then separated by centrifugation within 30 min. Blood lactate assay was then carried out immediately using “lactate oxidase - peroxidase” method in RANDOX Daytona Autoanalyser. HbA1c estimations were carried out in separately collected anticoagulated blood using “latex agglutination inhibition method” in RANDOX Daytona Autoanalyser. BMI was calculated using Quetelet’s index. Maternal age and blood pressure were noted. Data was collected regarding maternal age, obstetric score and consanguinity. They were followed up till delivery to know their gestational age at delivery, mode of delivery, fetal birth weight and any complications if they encountered.
Statistical Analysis
Microsoft office excel was used to compile data for statistical analysis. Data analysis was done with two softwares, namely, Epi Info version 3.5.4 and SPSS 16.0 by applying independent samples t-test and Pearson’s correlation test.
Results
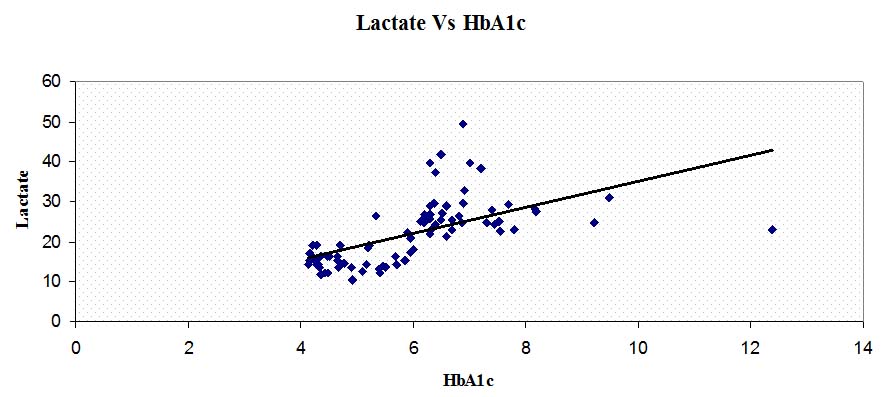
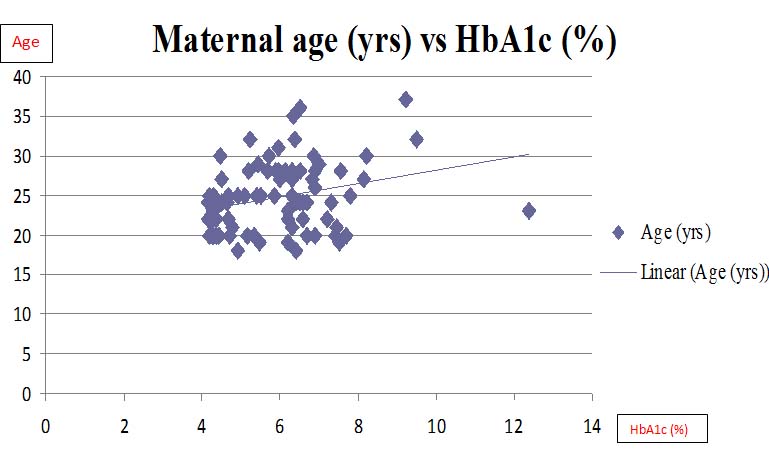
GDM mothers showed significantly higher lactate than normal pregnant women (p<0.001). GDM mothers also showed significantly higher HbA1c levels than normal pregnant women (p<0.001). Systolic (p<0.001) and diastolic blood pressures (p<0.001) and even the fetal birth weight (p<0.001) were significantly higher in GDM group than normal pregnancy group. Further, this increased HbA1c levels showed significant positive correlation with lactate (p<0.001), systolic BP (p<0.001), diastolic BP (p<0.001) and fetal birth weight (p<0.001). Though gravidity, consanguinity, gestational age at delivery and mode of delivery had a variation between the groups, they failed to show significance. [Table/Fig-1] summarizes the biochemical and clinical data obtained during the study. Correlations of HbA1c levels with lactate levels and maternal age are graphically depicted in [Tables/Fig-2,3].
Mean±SD of various parameters studied between GDM and NP groups., GDM – gestational diabetes mellitus; NP – normal pregnancy.
| No. of Subjects | Maternal age (yrs) | BMI (kg/m2) | SBP (mm Hg) | DBP (mm Hg) | HbA1c (%) | Lactate (mg/dl) | FBW (kg) |
|---|
| GDM group | 40 | 25.52±4.74 | 23.68±3.48 | 142.25±2.23* | 89.90±9.34* | 7.06±1.18* | 28.22±6.24 | 3.78±0.23* |
| NP group | 40 | 23.87±3.65 | 23.55±3.09 | 118.00±0.43* | 78.70±8.87* | 4.85±0.60* | 15.60±2.97* | 2.43±0.29* |
| p- value | | 0.085 | 0.8602 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
*significant
Showing correlation between lactate and HbA1c levels.
Lactate (mg/dl), HbA1c (%)
Showing correlation between HbA1c and maternal age.
HbA1c (%)
Discussion
In type 2 DM individuals, an increased proportion of plasma glucose undergoes non-oxidative glycolysis (thereby producing more lactate), representing a novel early abnormality in its pathogenesis. Further, this increase is linked to insulin resistance rather than insulin deficiency [7]. Lactic acidemia in type 2 DM patients was explained by reduction of renal excretion due to slight glomerular impairment. The median lactate levels were significantly higher in subjects with obesity with or without metabolic syndrome, compared with lean controls and this was explained by the lasting oxygen-deficient environment in them [8]. In those with severe diabetes, ~50% of circulating lactate and pyruvate was converted to glucose by liver. Interestingly, D-Lactic acid was shown to be converted to blood glucose more slowly or to a lesser extent than L-Lactic acid [9].
In pre-gestational DM (females with Type 2 DM, who later became pregnant), fetal blood obtained through cordocentesis revealed polycythemia and acidemia but not hypoxemia and that was said to be related to duration of diabetes. In GDM pregnancies (diabetes first detected during pregnancy), increased placental weight and size, decreased feto-placental ratio, altered placental morphology, villous immaturity and increased villous branching are reported. Further, fetal-placental beta cell hyperplasia is thought to explain the pathogenesis of GDM [5].
Lactate causes relaxation in placental vessels of normal pregnancies by an endothelium-independent, oxygen dependent process through the generation of hydrogen peroxide (H2O2) and stimulation of cGMP production. But this relaxation to lactate was significantly less in GDM placental vessels. The primary mechanism for these observations appeared to be an impaired response of placental vessels’ catalase activity to H2O2 and lactate rather than involving prostaglandin mediators. In GDM placental vessels, impairment in the cGMP-mediated relaxation to exogenous and endogenous H2O2 is proposed. Since, relaxation to lactate could be a potential defense mechanism of placental circulation to protect against fetal hypoxia, the loss of this mechanism in GDM could contribute to the complications [10].
Pregnant women with abnormal glucose metabolism exhibited higher rate of complications with rising HbA1c titre. The determination of HbAlC is thus, important in the screening, diagnosing and assessing the prognoses of the gestational abnormal glucose metabolism [11].
On analysis, our results show that mothers with GDM have significantly higher blood lactate and HbA1c levels than normal pregnant women. Thus, gestational diabetic women with heavily deranged carbohydrate metabolism would have had higher rates of anaerobic metabolism than normal pregnant women, which can be explained by the fact that, an increased proportion of plasma glucose undergoes glycolysis in GDM subjects following a mixed meal. This altered metabolic fate of plasma glucose might have been due to one or more of the following reasons [7]:
Differential resistance of individual metabolic pathways to stimulation by insulin.
Impaired insulin secretion.
Increased mass action effects of plasma glucose due to post-prandial hyperglycaemia.
Prior chronic hyperglycaemia.
Reduced systemic glucose disposal in GDM (and type 2 DM) has been shown to be accounted for by [7]:
Decreased glycogen synthesis.
Reduced glucose oxidation in spite of normal non-oxidative glycolysis of plasma glucose in GDM patients - so that latter accounted for a relatively greater proportion of overall plasma glucose disposal in GDM patients.
Limitation
The small sample size could be a limitation to the current study. Further, studies with more sample size, robust design and additional parameters of importance may help us to understand the subject better, which in turn helps obstetricians to handle GDM mothers safely.
Conclusion
Maternal blood lactate and HbA1c levels have a significant role to play in determining the metabolic mileau, in terms of carbohydrate metabolism of both mother and fetus and thus, their obstetric and general health outcomes and thus to decide whether to plan their pregnancies in case their blood lactate levels are then raised.
*significant
[1]. Mithal A, Bansal B, Kalra S, Gestational diabetes in India: Science and societyIndian J Endocrinol Metab 2015 19(6):701-04. [Google Scholar]
[2]. Nagalakshmi CS, Devaki RN, Akila P, Suma KB, Prashant V, Suma MN, Exploration of the clinico-biochemical parameters to explain the altered renal mechanisms in gestational diabetes mellitusJCDR 2012(Suppl-1) 6(3):369-71. [Google Scholar]
[3]. Prando R, Cheli V, Buzzo P, Melga P, Ansaldi E, Accoto S, Blood lactate behaviour after glucose load in diabetes mellitusActa Diabetologica 1988 25(3):247-56. [Google Scholar]
[4]. Metza L, Sirventa P, Pya G, Brunb JF, Fedoub C, Raynaudb E, Relationship between blood lactate concentration and substrate utilization during exercise in type 2 diabetic post-menopausal womenMetabolism 2005 54(8):1102-07. [Google Scholar]
[5]. Taricco E, Radaelli T, Rossi G, Santis MSND, Bulfamante GP, Avagliano L, Effects of gestational diabetes on fetal oxygen and glucose levels in vivoBJOG 2009 116:1729-35. [Google Scholar]
[6]. Radder JK, Van Roosmalen J, HbA1c in healthy, pregnant womenNeth J Med 2005 63(7):256-59. [Google Scholar]
[7]. Bokhari S, Emerson P, Israelian Z, Gupta A, Meyer C, Metabolic fate of plasma glucose during hyperglycaemia in impaired glucose tolerance: evidence for further early defects in the pathogenesis of type 2 diabetesAm J Physiol Endocrinol Metab 2009 296(3):E440-44. [Google Scholar]
[8]. Fang L, Jun-xi L, Jun-ling T, Li L, Hui-juan L, Xu-hong H, Relationship of plasma creatinine and lactic acid in type 2 diabetic patients without renal dysfunctionChin Med J 2009 122(21):2547-53. [Google Scholar]
[9]. Roger C, Meutter DE, Shreeve WW, Conversion of DL-Lactate-2-C14 or -3-c14 or pyruvate-2-c14 to blood glucose in humans: Effects of diabetes, insulin, tolbutamide and glucose loadJournal of Clinical Investigation 1963 42(4):525-33. [Google Scholar]
[10]. Figueroa R, Martinez E, Fayngersh RP, Tejani N, Mohazzab-H KM, Wolin MS, Alterations in relaxation to lactate and H2O2 in human placental vessels from gestational diabetic pregnanciesAm J Physiol Heart Circ Physiol 2000 278:H706-13. [Google Scholar]
[11]. Zhang XM, Ding YL, Glycosylated hemoglobin test in gestational abnormal glucose metabolismZhong Nan Da Xue Xue Bao Yi Xue Ban 2008 33(1):85-88. [Google Scholar]