Fatal Toxic Megacolon in a Child of Hirschsprung Disease
Shiwani R Garg1, Pragati A Sathe2, Annapurna C Taware3, Ketaki M Surve4
1 Fellow in Paediatric Pathology, Department of Pathology, Seth G.S.M.C and KEM Hospital, Mumbai, Maharashtra, India.
2 Assosiate Professor, Department of Pathology, Seth G.S.M.C and KEM Hospital, Mumbai, Maharashtra, India.
3 Assosiate Professor, Department of Pathology, Seth G.S.M.C and KEM Hospital, Mumbai, Maharashtra, India.
4 Fellow in Paediatric Pathology, Department of Pathology, Seth G.S.M.C and KEM Hospital, Mumbai, Maharashtra, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Shiwani R Garg, Flat No. 105, M2B, Pratiksha Nagar, Sion (E), 400022, India.
E-mail: shiwanig1011@gmail.com
Hirschsprung disease (HD) in late childhood is uncommon and often undiagnosed or misdiagnosed. However, in a patient with Hirschsprung disease, of greater significance is the occurrence of life threatening enterocolitis. In its more severe form, this is associated with gross dilatation of the colon and profound toxaemia, the combination being termed toxic megacolon. Because of its relative rarity, we report a case of 10-year-old child who had a history of chronic constipation for nine years. He later developed complications and presented to the emergency department with toxic megacolon, a rare occurrence due to neglected constipation. Though patient’s condition was unstable, laparotomy with right transverse colostomy was performed after appropriate intravenous rehydration. The dilated bowel loops were decompressed and intraoperatively multiple site biopsies were done. Histopathological examination of transition zone biopsy revealed absence of ganglion cells suggestive of Hirschsprung disease. But few hours later patient’s condition worsened and he succumbed.
Childhood, Chronic constipation, Enterocolitis
Case Report
A 10-year-old male child, reported to the emergency department with complaints of gradually progressing abdominal distension, loss of appetite and swelling over feet since five days. There was history of constipation with passing of hard stools since the age of six months. His birth details revealed a history of delayed passage of meconium (i.e., after 24 hours). No further investigations were done at that time or even by the age of six months. He was delivered vaginally at term. There was no developmental delay or mental retardation. On enquiry, it was discovered that patient was being symptomatically relieved by repeated enemas since six months of age without being investigated for constipation.
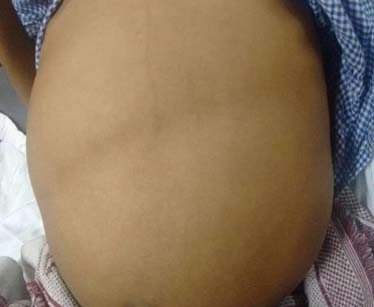
Examination revealed a sick, pale and dehydrated child with poor built. He also had bilateral pitting pedal oedema and clubbing (Grade III). His heart rate was 120 beats per min, blood pressure was 106/60mm Hg and respiratory rate was 30 per min. Gross abdominal distension was noted with visible bowel loops and umbilicus pointing downwards and transversely stretched [Table/Fig-1]. There was diffuse abdominal tenderness. On auscultation, sluggish bowel sounds were heard. On rectal examination, anal canal was empty and hard stool was palpable at the tip of the finger.
Abdominal distension with visible bowel loops.
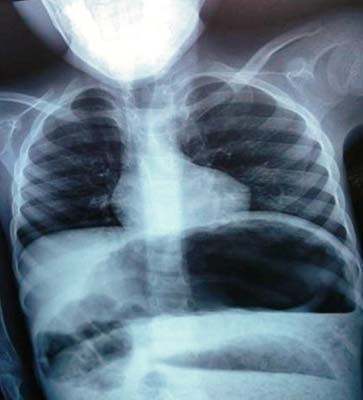
Laboratory investigations revealed haemoglobin - 3.9 gm%, total WBC count- 31100 cells/cumm, Differential count – polymorphs 94% and lymphocytes 6%. There was electrolyte imbalance with hyponatremia (126 mEq/l) and hyperkalemia (5.6 mEq/l). A radiograph of the abdomen revealed marked dilatation of the intestinal loops with air fluid levels in hypochondrium without perforation [Table/Fig-2]. The diagnosis of toxic megacolon was suggested with a suspicion of Hirschsprung disease. Though patient’s condition was unstable, laparotomy with right transverse colostomy was performed after appropriate intravenous rehydration. The dilated bowel loops were decompressed and about five litres of fecal matter was evacuated. Also, intraoperatively, biopsies were taken from transition zone, a site 10 cm proximal to the transition zone and the colostomy site.
Radiograph of the abdomen showing marked dilatation of the intestinal loops with air fluid levels in hypochondrium.
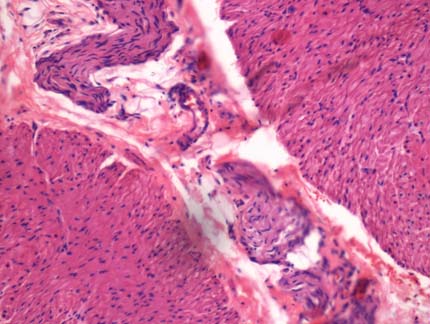
On histopathological examination, biopsy from the transition zone revealed absence of ganglion cells in myenteric plexus with hypertrophied nerve bundles [Table/Fig-3], whereas the biopsies taken 10 cm proximal to transition zone and the colostomy site revealed ganglion cells in the submucosal and myenteric plexus. Hence, the diagnosis of Hirschsprung disease was rendered.
Biopsy from the transition zone showing absence of ganglion cells in myenteric plexus with hypertrophied nerve bundles. (H&E, x 400).
Twelve hours after the operative procedure, patient’s condition worsened and he succumbed. An autopsy was requested to confirm the diagnosis of toxic megacolon.
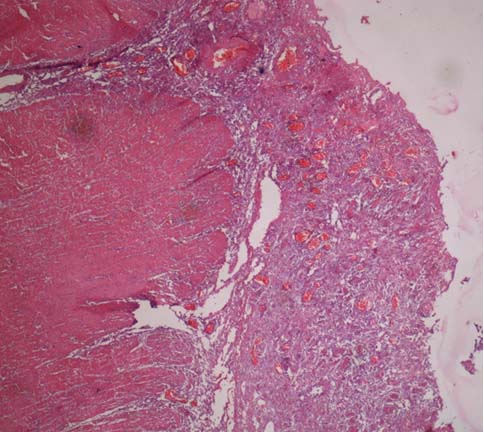
At autopsy, the entire large intestine showed marked dilatation with a maximum circumference of upto 30 cm [Table/Fig-4]. The colonic wall was significantly thickened upto one cm. The mucosa of the colon was oedematous, congested, and shaggy and had multiple ulcers [Table/Fig-5]. The ulcers were large, shallow (depth upto submucosa) with irregular margins, sloping edges and necrotic base suggesting a diagnosis of enterocolitis. On histopathology, hypertrophied nerve bundles without ganglion cells were identified in the sub mucosal and myenteric plexuses in the distal rectum. The proximal dilated colon showed extensive ulceration of the mucosa with diffuse mixed inflammatory infiltrate in the mucosa and submucosa [Table/Fig-6]. The submucosa was oedematous. The submucosal and myenteric plexuses showed presence of ganglion cells. No hypertrophied nerve bundles were seen. The serosa showed presence of organised inflammation. Kidneys showed features of tubular damage. The other organs did not show any abnormality.
At autopsy, the entire large intestine showing marked dilatation.

Colonic mucosa is oedematous, shaggy and congested with multiple ulcers.

Section from the proximal dilated colon shows extensive ulceration of the mucosa with crypt abcesses and diffuse mixed inflammatory infiltrate in the mucosa and submucosa (H&E, x 100).
Discussion
Hirschsprung disease (HD) affects approximately 1 in 5,000 live births and usually presents in infancy and early childhood [1]. The presentation of HD varies in relation to age and extent of the disease. In the developed countries, majority of HD cases (90%) are diagnosed in the neonatal period [2]. The challenges in the developing world are delayed presentation with cases presenting late in childhood, adolescence or even in adults [3,4]. Sharma et al., reported that the reasons for delayed presentation in such cases could be poverty, ignorance, illiteracy and long distances to travel for medical help [5].
In HD due to defective migration of ganglion cell precursors of the neural crest into the hindgut, there is total absence of the ganglion cells in the submucosal and myenteric plexuses of the affected portion of the large intestine [1]. Defects in neural crest stem cell function have been linked to HD by some recent molecular studies [6]. In HD, the aganglionic segment remains contracted and the proximal segment has preserved peristaltic function; due to which there is work hypertrophy, dilatation of the normally innervated colon. Sometimes even perforation can also occur [1]. In HD the aganglionic segment begins and extends proximally from the anus [7]. The rectosigmoid region of the colon is affected in short segment disease; whereas long segment disease can affect the entire colon. Both small and large intestines are affected rarely in HD [8].
The symptoms vary with the age of the patient and the extent of the disease. In the newborn period, bilious emesis, abdominal distension, failure to pass meconium or abnormal stool frequency is common. In undiagnosed cases, there may be frequent episodes of fecal impaction or acute life threatening enterocolitis. The latter develops in 15% to 50% cases of HD and may be the presenting feature in up to 12% patients. It remains as the main cause of death and the mortality rate can be as high as 20-50% [9]. The disease pathogenesis is still unclear, but evidences suggest that the intestinal microbiota may play an important role in the development of HD and Hirschsprung associated enterocolitis (HAEC) [10]. Recently, bacteria and viruses have been shown to be involved in the pathogenesis of HAEC. The infection and colonization by specific intestinal bacteria may be harmful to the intestinal barrier, microenvironment and the immune responses, leading to recurrent HAEC [11]. A recent study has reported that probiotics significantly diminish the incidence and decrease the severity of HAEC [12].
Toxic megacolon is defined as an acute dilatation of the colon associated with clinical evidence of toxaemia. Leading symptoms are abdominal distension, constipation, reduced bowel sounds and constitutional symptoms such as fever, tachycardia, or hypotension supported by radiology. Although this is most frequently associated with ulcerative colitis, it may also occur in association a variety of other conditions, including crohn’s disease, amoebic colitis, pseudomembranous colitis, cholera, and bacillary dysentery. TM (Toxic Megacolon) complicating HD is quite rare [13].
Rectal biopsy is considered the gold standard for diagnosis and may be supported by findings on abdominal radiographs, contrast enema, or anorectal manometry. Each of these procedures has advantages and disadvantages related to availability, technical expertise, radiation exposure and invasiveness [14]. The association between the congenital absence of colonic ganglion cells and an increased acetylcholinesterase (AChE) expression in the affected tissue is also of diagnostic importance in Hirschsprung’s disease. Normal nerves do not stain for AChE, but increased AChE expression is associated with the hypertrophied extrinsic nerve fibres of the aganglionic segment in HD [15].
The principle of management of HD is the removal of the aganglionic portion and a “pull-through” of the proximal ganglionated bowel, first described by Swenson and Bill in 1948 [16]. Later, Duhamel and Soave described the retrorectal pull-through and endorectal pull-through respectively. These three procedures have been widely used in the surgical management of this condition and the outcome and prognosis has been very good and comparable among the three procedures [17].
It is important to remember that HD does not present late, it is the diagnosis that is missed. In any child or young adult with constipation, a history of delayed or non passage of meconium after birth should be elicited. A rectal biopsy should be performed to exclude HD.
Conclusion
Constipation is a common childhood problem. HD should not be forgotten as a cause of constipation at any age. Active steps should be taken to diagnose the disease so that serious and life threatening complications do not occur.
[1]. Roy CC, Silverman A, Alagille D, Congenital aganglionic megacolon (Hirschsprung’s disease). In: Roy CC, Silverman A, Alagille D, editorsPediatric clinical gastroenterology 1995 4th edSt. Louis (MO)Mosby:503-15. [Google Scholar]
[2]. Singh SJ, Croaker GD, Manglick P, Wong CL, Athanasakos H, Elliott E, Hirschsprung’s disease: The Australian Paediatric Surveillance Unit’s experiencePediatr Surg Int 2003 19:247-50. [Google Scholar]
[3]. Ekenze SO, Ngaikedi C, Obasi AA, Problems and outcome of Hirschsprung’s disease presenting after 1 year of age in a developing countryWorld J Surg 2011 35:22-6. [Google Scholar]
[4]. Bandré E, Kaboré RA, Ouedraogo I, Soré O, Tapsoba T, Bambara C, Hirschsprung’s disease: Management problem in a developing countryAfr J Paediatr Surg 2010 7:166-68. [Google Scholar]
[5]. Sharma S, Gupta DK, Hirschsprung’s disease presenting beyond infancy: Surgical options and postoperative outcomePediatr Surg Int 2012 28:5-8. [Google Scholar]
[6]. Iwashita T, Kruger GM, Pardal R, Kiel MJ, Morrison SJ, Hirschsprung disease is linked to defects in neural crest stem cell functionScience 2003 301:972-76. [Google Scholar]
[7]. Hirschsprung’s disease: congenital megacolon. In: Feldmen M, Friedman LS, Sleisenger MH, editorsSleisenger & Fordtran’s Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management 2002 7th edPhiladelphia, PASaunders:2131-5. [Google Scholar]
[8]. Stewart DR, von Allmen D, The genetics of Hirschsprung diseaseGastroenterol Clin North Am 2003 32:819-37. [Google Scholar]
[9]. Chumpitazi B, Nurko S, Pediatric Gastrointestinal Motility Disorders: Challenges and a Clinical UpdateGastroenterol Hepatol (N Y) 2008 4:140-48. [Google Scholar]
[10]. Yan Z, Poroyko V, Gu S, Zhang Z, Pan L, Wang J, Characterization of the intestinal microbiome of Hirschsprung’s disease with and without enterocolitisBiochem Biophys Res Commun 2014 445:269-74. [Google Scholar]
[11]. Poroyko HL, Hirschsprung disease and the intestinal microbiomeClin Microbiol 2014 3:172 [Google Scholar]
[12]. Wang X, Li Z, Xu Z, Wang Z, Feng J, Probiotics prevent Hirschsprung’s disease-associated enterocolitis: a prospective multicenter randomized controlled trialInt J Colorectal Dis 2015 30:105-10. [Google Scholar]
[13]. Khasanov R, Schaible T, Wessel LM, Haql Cl, The Surgical Treatment of Toxic Megacolon in Hirschsprung DiseasePaediatr Emer Care 2015 (Epub ahead of print) [Google Scholar]
[14]. De Lorijn F, Reitsma JB, Voskuijl WP, Aronson DC, Ten Kate FJ, Smets AM, Diagnosis of Hirschsprung’s disease: a prospective, comparative accuracy study of common testsJ Pediatr 2005 146:787-92. [Google Scholar]
[15]. Moore SW, Johnson G, Acetylcholinesterase in Hirschsprung’s diseasePediatr Surg Int 2005 21:255-63. [Google Scholar]
[16]. Swenson O, Bill AH, Resection of the rectum and rectosigmoid with preservation of the sphincter for benign spastic lesions producing megacolonSurgery 1948 24:212-20. [Google Scholar]
[17]. Mabula BJ, Kayange NM, Manyama M, Chandika AB, Rambau PF, Chalya PL, Hirschsprung’s disease in children: a five year experience at a University teaching hospital in northwestern TanzaniaBMC Research Notes 2014 7:410 [Google Scholar]