Oral Submucous Fibrosis (OSMF) is defined as “An insidious chronic disease affecting any part of the oral cavity and sometimes the pharynx. Although occasionally preceded by and/or associated with vesicle formation, it is always associated with a juxta-epithelial inflammatory reaction followed by a fibroelastic change of the lamina propria with epithelial atrophy leading to stiffness of the oral mucosa and causing trismus and inability to eat” [1]. Ramanathan K in his study hypothesized that this condition was an Asian version of sideropenic dysphagia wherein the chronic deficiency of iron may lead to mucosal susceptibility to irritants such as chilli and areca nut use. Iron deficiency causes reduction in myoglobin, cytochromes and other enzymes present in different tissues [2]. According to Huang S et al., in the process of collagen synthesis, iron gets utilized, by the hydroxylation of proline and lysine, leading to decreased serum iron level [3].
According to Murti PR et al., the presence of palpable bands is a diagnostic criterion for this disease, and the incidence of malignant change in these patients ranges from 2% to 10%. About 2.5 million individuals are affected worldwide, with most cases being in Southern India [4]. It also occurs in migrant chewers of betel quid in other countries.
Considering the multifactorial aetiology, more research is required to develop sensitive, specific, and faster tests in the diagnosis and prognosis of these diseases. The diagnostic and prognostic value of iron in malignancies like oesophageal cancers (Plummer Vinson syndrome), oral carcinoma, post-cricoidal carci-noma, and oesophageal carcinoma is well recognized. Recently trace element like iron is receiving much attention in the detection of oral cancer and precancerous lesions or conditions as it was found to be significantly altered in these conditions.
There is a need to study that whether the alteration in the serum iron and haemoglobin level leads to initiation of OSMF in the absence of betel nut or tobacco chewing habit or they accelerate the disease process already initiated by betel nut or tobacco chewing. Thus, there is a need to understand the aetiopathogenesis and progression of these conditions. Hence, this study was performed to determine the serum iron level in all the groups and also to comprehend the association of the levels of haemoglobin and serum iron in different stages of OSMF.
Materials and Methods
The present cross-sectional observational study was conducted on 120 patients in the age group between 16-65 years, selected amongst those who reported to the Oral Medicine and Radiology Department of Goa Dental College and Hospital, Bambolim, Goa, India, from June 2012 to December 2013. The study was approved by the Ethical Committee, Goa Dental College and Hospital, Bambolim, Goa. Patients were grouped as follows:
Group I – A total of 40 healthy individuals (20 males and 20 females) so as to have a standard measurement against which the other two groups will be compared i.e., this group will have normal serum iron and haemoglobin values.
Group II – A total of 40 patients with OSMF (33 males and 7 females).
Group III – A total of 40 patients with iron deficiency anaemia (20 males and 20 females).
Formula to Calculate Sample Size:
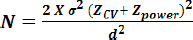
The sample size was calculated using quantitative data in Ganapathy KS et al., study (conducted on population of Bangalore) as no categorical data was available regarding the prevalence of OSMF in the population of Goa [10]. The sample size was calculated for each group (i.e., Group A, B and C) was 31 subjects (based on 99.99 % confidence interval) but taking into consideration the dropout rate of 10% of subjects in a study, the sample size was decided to be 40 subjects for each group.
Inclusion Criteria
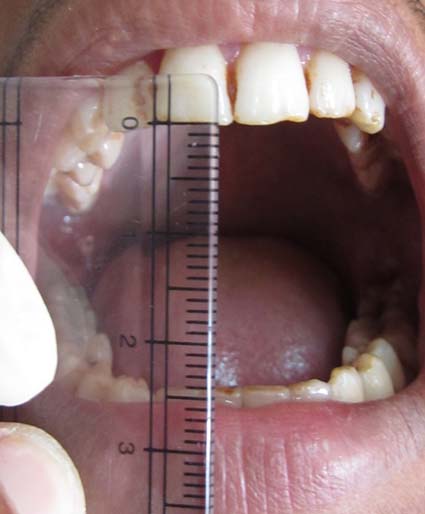
Patients in Group A included healthy individuals with no oral lesions and no history of tobacco or areca nut chewing or alcohol intake. Patients in Group B with OSMF were clinically diagnosed and graded [Table/Fig-1] by using the criteria as mentioned by Khanna JN and Andrade NN [5]. Patients in Group C included patients with iron deficiency anaemia clinically (patients who had history of generalized fatigue and weakness and signs of pallor in sclera and mucosa were included) and biochemically diagnosed with iron deficiency anaemia by detecting serum iron levels.
Restricted mouth opening in oral submucous fibrosis.
Normal reference values for serum iron: 55-175 μg/dl (males); 50-170 μg/dl (females).
Exclusion Criteria
Oral mucosal lesions other than OSMF observed clinically.
Patients who have already been treated for OSMF.
Patients with systemic disorders like diabetes, hypertension, jaundice, liver/kidney disorders, and/or under treatment for any other systemic disease(s) in case of OSMF and control group.
Patients who were pregnant or on oral contraceptives.
Patients in the control group with any history of tobacco or areca-nut chewing or alcohol intake.
Patients unwilling to participate in the study.
Methods of Sample Collection
All the patients fulfilling the above criteria were informed about the study in their own mother tongue and only those who agreed and gave a written signed voluntary consent were enrolled in the study. All the enrolled subjects were then interviewed and examined in the dental clinic using clinical examination tools after recording case history. After satisfying the diagnostic criteria patients were subjected to the serum iron and haemoglobin estimation investigations. Approximately 5ml of venous blood was obtained by venipuncture of the median cubital vein after taking all aseptic precautions. Approximately 2ml of blood obtained was transferred to an Ethylene di amine tetra acetate (EDTA) coated clot activator bulb for haemoglobin estimation. Approximately 3ml of blood was allowed to clot for one hour in the plain bulb and was then centrifuged at 3000 rpm for 10 minutes to provide serum. This serum was preserved in a frozen state and was analyzed for serum iron estimation within one hour of its collection.
Estimation of Iron and Haemoglobin Values
Estimation of iron was done using Ferrozine method and haemoglobin by Sahli’s method. All the test-tubes used for analysis were kept immersed overnight in deionized water and then washed the next day using deionized water. The serum sample used for the estimation was mixed with appropriate proportions of buffer and colour reagents supplied in the iron estimation kit in clean dry test-tube as per the manufacturer’s instructions. The absorbance of these samples was compared to that of the standard solution provided in the kits at 578nm in a semi-autoanalyzer (Microlab – 200). For Sahli’s method, approximately 0.2N HCl was put into the graduated test tube to mark 2gm. Blood sample of 0.02ml was then transferred into the solution with the help of a pipette and mixed quickly. The tube was placed into the tube holder of the Sahli’s haemometer.
Statistical Analysis
The mean values and standard deviations for all the groups were calculated. Kruskal-Wallis test was used to compare more than two means simultaneously, i.e., whether there was a significant difference between the mean values of serum iron and haemoglobin amongst the three study groups. Mann-Whitney test was used for multiple comparisons between any two groups if Kruskal-Wallis test was found to be significant. Students t-test was applied to determine whether significant difference existed in the serum iron and haemoglobin levels of subgroups of OSMF (Group B). Pearson correlation coefficient was applied to assess any significant relation in serum iron and haemoglobin values in the OSMF group.
Results
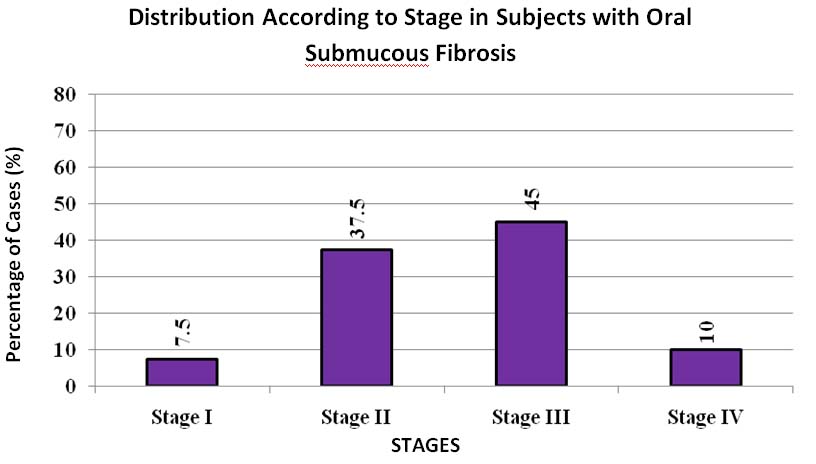
The study (OSMF) group comprised of 40 new cases of OSMF ranging from 16 to 65 years in age with a mean age of 35.92±11.08 years. The majority of cases were between 16 to 25 years. The OSMF group showed male predominance with 33 males and 7 females. The distribution of subjects with OSMF according to stages showed the maximum people were in stage II and stage III [Table/Fig-2] as per Khanna JN and Andrade NN [5]. The institutional incidence of OSMF in our study was 0.22% based on number of patients reporting to our institution i.e., 40 patients diagnosed with OSMF out of total 18,000 patients reported in the hospital OPD within one and a half year duration of study. It was observed that OSMF is mostly seen in younger individuals (the maximum numbers of patient reported were in the age group of 16-25 years) as compared to iron deficiency anaemia group (the maximum numbers of patient reported were in the age group of 46-55 years).
Distribution of subjects with OSMF according to stages as per Khanna JN and Andrade NN [5] (Group B).
It was also observed that subjects in iron deficiency anaemia group did not show presence of OSMF in the absence of chewing habits of gutkha and/or related products. The majority of OSMF patients showed iron deficiency anaemia i.e., 25/40 subjects (62.5%), 8/40 subjects (20%) showed only low serum iron and normal haemoglobin level (iron deficient individuals), 3/40 subjects (7.5%) showed normal serum iron but low haemoglobin (anaemia due to deficiency of other nutrients like Vitamin B12 and folic acid) and 4/40 subjects (10%) showed normal serum iron and normal haemoglobin levels [Table/Fig-3].
Distribution of subjects according to serum iron and haemoglobin status in the subgroups of study group B.
| Stages | Normal Serum Iron & Hb | Low Serum Iron & Normal Hb | Low Serum Iron & Hb (Iron Def An.) | Normal Serum Iron & Low Hb |
|---|
| Group B-I(No. of Cases) | 1(33.33%) | 1(33.33%) | 1(33.33%) | - |
| Group B-II(No. of Cases) | 2(13.33 %) | 2(13.33%) | 9(60%) | 2(13.33%) |
| Group B-III(No. of Cases) | 1(5.5%) | 4(22.22%) | 13(72.2%) | - |
| Group B-IVa(No. of Cases) | - | 1(25%) | 2(50%) | 1(25%) |
| Total | 4(10%) | 8(20%) | 25(62.5%) | 3(7.5%) |
Hb-Haemoglobin
Mean value of serum iron level of control group was 78.00±21.07mcg/dl; whereas, that of OSMF group was 34.06±20.67mcg/dl. On comparison of the OSMF group with the healthy group, OSMF group showed significantly lower level of serum iron with p< 0.001. Mean value of serum iron level in the anaemic group was 39.02±12.13mcg/dl, and in OSMF group was 34.06±20.67mcg/dl. On comparison between anaemic and OSMF group, the serum iron level was significantly lower in the OSMF group, with p<0.03 [Table/Fig-4].
Comparison of serum iron levels between the three study groups (Mann- Whitney test).
| Comparison Between Groups | No. of Patients | Mean Serum Iron level±SD (μg/dl) | z Value | p value |
|---|
| Group A (Control) &Group B (Oral Submucous Fibrosis) | 4040 | 78.00±21.0734.06±20.67 | 6.71 | <0.01S |
| Group A (Control) & Group C (Iron Deficiency Anaemia) | 4040 | 78.00±21.0739.02±12.13 | 7.59 | <0.01S |
| Group B (Oral Submucous Fibrosis) & Group C (Iron Deficiency Anaemia) | 4040 | 34.06±20.6739.02±12.13 | 2.16 | 0.031S |
S- Significant
Mean value of haemoglobin of control group was 13.83±1.39g/dl whereas that of OSMF group was 10.94±2.95gm/dl. On comparison of the OSMF group with the healthy group, OSMF group showed significantly lower levels of haemoglobin with p< 0.001. Mean value of haemoglobin in the anaemic group was 8.66±2.15 gm/dl whereas that of the OSMF group was 10.94±2.95gm/dl. On comparison between anaemic and OSMF group, the difference was statistically significant with p<.002 [Table/Fig-5].
Comparison of haemoglobin levels between the three study groups (Mann – Whitney test).
| Comparison Between Groups | No. of Patients | Mean Haemoglobin level±SD (gm/dl) | Z Value | p value |
|---|
| Group A (Control) &Group B (OSMF) | 4040 | 13.83±1.3910.94±2.95 | 4.49 | <0.01S |
| Group A (Control) & Group C (Iron Deficiency Anaemia) | 4040 | 13.83±1.398.66±2.15 | 7.69 | <0.01S |
| Group B (OSMF) & Group C (Iron Deficiency Anaemia) | 4040 | 10.94±2.958.66±2.15 | 3.06 | 0.002S |
S- Significant
Inter-stage comparison showed significantly lower haemoglobin and serum iron levels in stage III OSMF (Hb%= 10.19±2.85gm/dl/serum, Iron= 28.70±16.32mcg/dl) than stage II OSMF (Hb%= 10.75±2.88/dl, serum iron= 39.90±23.65mcg/dl) with p<0.12. On analysing the values by Pearson’s correlations, it was observed that values of haemoglobin level had significant correlation with serum iron levels in the OSMF stage III (correlation value: 0.511) [Table/Fig-6,7 and 8].
Mean, Standard Deviation & Standard Error Mean of Serum Iron in Subgroups of Oral Submucous Fibrosis Group.
| Sr. No. | Parameter | Sample Size | Mean | Std. Deviation | Std. Error Mean |
|---|
| 1 | STAGE B-I | 3 | 40.33 | 12.27 | 7.07 |
| 2 | STAGE B-II | 15 | 39.90 | 23.65 | 6.11 |
| 3 | STAGE B-III | 18 | 28.70 | 16.32 | 3.85 |
| 4 | STAGE B-IV | 4 | 27.45 | 29.96 | 14.98 |
Mean, Standard Deviation & Standard Error Mean of Haemoglobin in Subgroups of Oral Submucous Fibrosis Group B.
| Sr. No. | Parameter | Sample Size | Mean | Std. Deviation | Std. Error Mean |
|---|
| 1 | STAGE B-I | 3 | 14.03 | 4.01 | 2.31 |
| 2 | STAGE B-II | 15 | 10.75 | 2.88 | 0.74 |
| 3 | STAGE B-III | 18 | 10.19 | 2.85 | 0.67 |
| 4 | STAGE B-IV | 4 | 10.10 | 2.15 | 1.07 |
Pearson’s correlation coefficient in Subgroups of Oral Submucous Fibrosis (group B).
| Group | No. of Cases | Significance | Pearson correlation coefficient |
|---|
| Stage B-I Oral Submucous Fibrosis | 3 | 0.604 | 0.583 |
| Stage B-II Oral Submucous Fibrosis | 15 | 0.860 | 0.073 |
| Stage B-III Oral Submucous Fibrosis | 18 | 0.030 | 0.511 |
| Stage B-IV Oral Submucous Fibrosis | 4 | 0.909 | 0.091 |
Discussion
OSMF has been explained as a chronic progressively scarring disease of the oral cavity. Until recently, it was thought to be localized to the Indian subcontinent, China and other regions of Asia but is now considered to be of global importance due to large number of migrant populations also demonstrating the condition. OSMF is a well-recognized entity having a potential for malignant transformation. Therefore, the study of oral premalignant conditions is of importance for the prevention of oral cancer because these conditions may be treated to prevent their progression to oral cancer or used as surrogate (intermediate) markers for oral cancer intervention.
The role of iron in OSMF was first reviewed by Moos KS and Madan DK [6]. Recently, trace elements have been receiving much attention in the detection of oral cancer and precancer as they have been found to be significantly altered in head-neck, lung, and breast carcinomas. There is a need to assess whether iron has any modifying effect in the aetiology of OSMF, since only few studies have been conducted worldwide to find out its role in OSMF [7–14].
It was observed that the serum iron levels in the case of OSMF patient were lower than the iron deficiency anaemia. it can be attributed to the fact that increased collagen formation in OSMF utilizes more serum iron and hence the value is observed lower than the iron deficiency anaemia patient. A statistically significant difference was noted between mean values of serum iron in OSMF and iron deficiency anaemia, when compared with the control and with each other (p<0.05). The results obtained in our study are in accordance to other studies [7–14].
It was observed that the haemoglobin level in case of OSMF patient was higher than that of the iron deficiency anaemia patients owing to the fact that all patients in the group (OSMF) did not have lower haemoglobin level than the normal range (normal range for haemoglobin in adult males = 13.0-17.0gm/dl and normal range for haemoglobin in adult females = 12.0-15.0gm/dl) whereas, haemoglobin was significantly low in every subject of iron deficiency anaemia group. A statistically significant difference was noted between mean values of haemoglobin in OSMF and iron deficiency anaemia, when compared with the control and with each other (p<0.05). The results obtained in our study are in accordance to the studies conducted by Ganapathy KS et al., [10], Rupak S. et al., [11], Karthik H et al., [12] and Taneja L et al., [15]. It was observed that though there was decrease in serum iron and haemoglobin concentration as clinical stage increased but the difference was statistically not very strongly significant. The reason can be, that there was not enough evidence to prove the relation significant and by increasing the sample size in the respective stages it can be achieved. The results obtained in our study are in accordance to the studies conducted by Ganapathy KS et al., [10], Rupak S et al., [11] and Tadakamadla J et al., [16].
Pearson’s correlation coefficient was applied to the data to find if any significant correlation exists and haemoglobin between serum iron in the four subgroups of OSMF. There was a statistically significant moderate correlation between mean values of serum iron and haemoglobin in Stage B-I OSMF (r = 0.583) and Stage B-III OSMF (r = 0.511) and weak statistically significant correlation in Stage B-II (r = 0.073) and Stage B-IV (r = 0.09) of OSMF. The results obtained in our study are in accordance to the study done by Karthik H et al., [12]. It thus, signifies that there exists a correlation in serum iron and haemoglobin values in various stages of OSMF. It implies that with the increase in severity of the disease the serum iron and haemoglobin levels are also progressively decreasing indicating poor prognosis.
Serum iron levels are considered as biochemical indicators for nutritional assessment and their low value can indicate the progress from oral precancer to oral cancer stage [17]. In our study, serum iron level in OSMF group was low in comparison to the control and iron deficiency anaemia group. This decrease in serum iron levels was progressive from control to iron deficiency anaemia group to OSMF group. It can be explained by the fact that gutkha and its related products contains fine grains of areca nut and ground tobacco which cause mechanical injury to oral tissues and leads to inability to take an adequate diet. Low socioeconomic status and the lack of adequate diet may result in iron, Vitamin B12 and folic acid deficiencies all of which can affect the oral mucosa therefore leading to low Serum iron level in the body.
Cytochrome is an iron dependent enzyme and plays a role in the normal maturation of epithelium and in state of iron deficiency, leads to epithelial atrophy. As a result, the oral mucosa gets more prone to attack of soluble irritants and enhances the collagen production due to stimulation of bucaal mucosal fibroblasts [18]. The deficiency of iron also results in improper vascular channel formation in oral mucosa leading to decreased vascularity. The cumulative effect of these factors leads to derangement in the inflammatory-reparative response of the lamina propria resulting in defective healing and scarification and fibrosis [14]. Another role of iron is in hydroxylation of amino acid ‘hydroxyproline’ which in its hydroxylated form is only found in collagen. This hydroxylation reaction requires ferrous iron and ascorbic acid. The increase in production of highly cross linked insoluble collagen type I utilizes iron which further leads to decrease in serum iron level [8].
It has been well observed in literature that the subjects with severe iron deficiency condition are at a greater risk of developing oral, post-cricoidal and oesophageal carcinoma [19]. Though OSMF is a clinically benign condition, it is a potentially malignant disease having a malignant transformation rate of 2 to 10% [20]. The increased prevalence of oral precancerous and cancerous lesions can only be reduced by proper education, particularly by increasing awareness of the deleterious effects of these habits, especially among the socially and economically weaker sections of society.
A significant correlation was found in serum iron and haemoglobin value in various subgroups of OSMF group. Therefore as the serum iron and haemoglobin level depletes in OSMF group stages, the severity of disease increases. So it can be used as an auxillary test in assessment of prognosis of the disease.
The low levels of haemoglobin level and normal serum iron can also be due to the deficiency of Vitamin B12 and folic acid therefore their estimation is also important in case when patients have normal serum iron but lower haemoglobin value. Hence, it is suggested that these parameters should also be examined to assess the effects caused by them in OSMF. This study can be modified further to include total iron binding capacity and serum ferritin as they can also serve as indicators for determining serum iron levels.
Limitation
The sample size if increased in each stage of OSMF may yield a statistically stronger correlation between the serum iron and haemoglobin values.
Conclusion
It was evident from present study that differences exist between mean serum iron and haemoglobin level in OSMF, iron deficiency anaemia, and control patients. The iron deficiency anaemia group was not found to be suffering from OSMF in the absence of habit, but OSMF patients were found to be with either normal serum iron and haemoglobin levels or suffering from iron deficiency. It also showed that there is a progressive decrease in serum iron and haemoglobin levels from stage I of OSMF to the stage IV of OSMF clinically. A significant correlation was found in serum iron and haemoglobin value in various subgroups of OSMF group. Therefore, as the level of serum iron and haemoglobin level depletes in the OSMF group, the severity/stages of disease increases. However, more studies are still required on a large scale to reveal role of serum iron role in OSMF.
Hb-Haemoglobin
S- Significant
S- Significant