Radiological and Histopathological Outcome of Giant Cell Tumor of Femur with Denosumab Treatment: A Case Report
Preethi Dileep Menon1, R. Krishnakumar2, Annie Jojo3
1 Intern, Department of Pathology, Amrita Institute of Medical Sciences, Kochi, Kerala, India.
2 Associate Professor, Department of Orthopedics, Amrita Institute of Medical sciences, Kochi, Kerala, India.
3 Professor, Department of Pathology, School of Medicine, Amrita Institute of Medical Sciences, Kochi, Kerala, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Preethi Dileep Menon, Intern, Department of Pathology, Amrita Institute of Medical Sciences, Kochi-682041, Kerala, India.
E-mail: preethi.d.m@gmail.com
Giant Cell Tumour of Bone (GCTB) is a benign but locally aggressive osteolytic skeletal neoplasm of young adults consisting of giant cells expressing RANK (Receptor Activator of Nuclear Factor-κB) and mesenchymal spindle-like stromal cells expressing RANKL (RANK ligand). The interaction of these cells leads to bone resorption. Recently, the RANKL inhibitor, denosumab, has demonstrated activity against giant-cell tumours. The current article reports a case of a Giant cell tumour of left distal femur with pathological fracture. A 34-year-old male patient presented with history of on and off dull aching pain in the left knee for 4 months followed by a history of trivial fall. He sustained a closed injury in the left knee, following which he was unable to bear weight and developed pain and swelling in left knee. Conventional radiographs and Computerized tomography (CT) was done which showed the presence of a left distal femoral osteolytic lesion and a histological analysis of a biopsy specimen confirmed the diagnosis of GCTB. The patient was treated with neoadjuvant denosumab therapy which resulted in successful downstaging of the tumour followed by extended curettage of the lesion with high speed burr and argon laser cautery. The post-curettage microscopic examination revealed the absence of osteoclast-type giant cells.
Giant cell tumour of bone, Receptor activator of nuclear factor-κB ligand, Osteoclast like giant cells, New bone formation
Case Report
A 34-year-old male patient came with complaints of left knee pain following a trivial fall which was assessed at a local hospital on the same day and presented to our hospital the next day. He was unable to bear weight and developed pain and swelling in left knee. He also complained of on and off dull aching pain in the left knee for 4 months prior to the fall. There was no associated history of fever, chest pain or other joint swelling. He had not taken any kind of treatment prior to presentation. Physical examination of the left knee revealed a grossly swollen knee with focal tenderness to palpation in the distal femur and inability to move the knee due to severe pain.
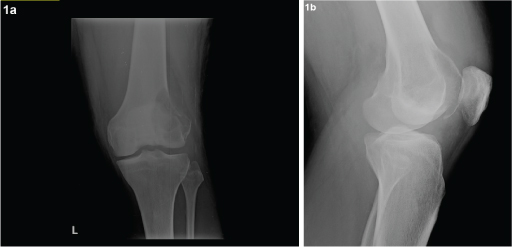
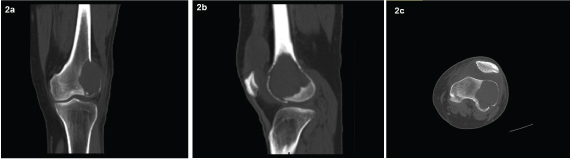
Anteroposterior (AP) and lateral radiographs of the left knee showed a lytic lesion in the left lateral femoral condyle [Table/Fig-1a&b]. Calcium, phosphorus, vitamin D and parathyroid hormone levels were done and found to be within normal limits. Complete blood count and chemistry panel including chest X-ray and CT chest was done and did not show any abnormalities or lesions. Multidetector computed tomography (MDCT) of knee showed a 3.8 (TR) x 6.8(CC) x 4.2(CC)cm epiphyseal, eccentric, sub-articular lytic lesion with narrow zone of transition and cortical break involving the articular surface noted in the left lateral femoral condyle [Table/Fig-2a-c]. Magnetic resonance imaging (MRI) knee was done and it confirmed the presence of the lesion.
Anteroposterior (AP) and lateral radiographs of the left knee showing lytic lesion of lateral femoral condyle of left femur.
CT showing lytic lesion in the coronal, sagittal and axial planes prior to Denosumab treatment.
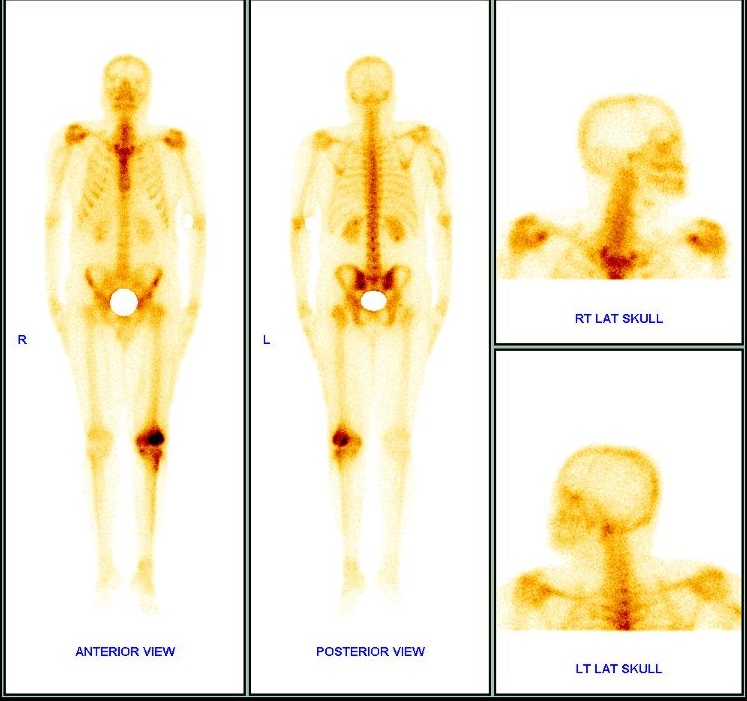
Left femoral condyle Giant Cell Tumour (GCT) was suspected with cortical break and peri-arterial soft tissue extension. In addition, whole body skeletal scan done showed increased Technetium 99m-methyl diphosphonate (99m Tc MDP) seen in lateral femoral condyle of left femur with no evidence of any distant skeletal lesions [Table/Fig-3].
Increased 99m Tc MDP seen in lateral femoral condyle of left femur- site of known pathology. No evidence of any distant skeletal lesions.
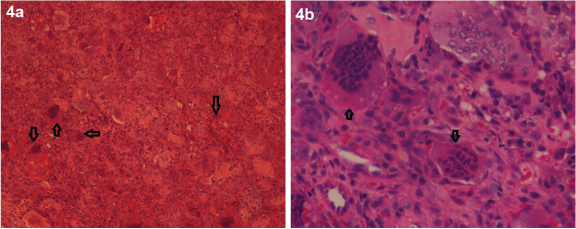
An incisional biopsy of the left lateral femoral condyle was performed, and analysis of the specimen revealed bony trabeculae with a neoplasm composed of giant cells separated by mononuclear cells arranged in diffuse sheets leading to a diagnosis of GCTB [Table/Fig-4a&b].
(H&E stain, 10X and 40X) Histology of the biopsy specimen before the start of denosumab treatment showed bony trabeculae with a neoplasm composed of giant cells separated by mononuclear cells arranged in diffuse sheets which is consistent with the diagnosis of GCTB.
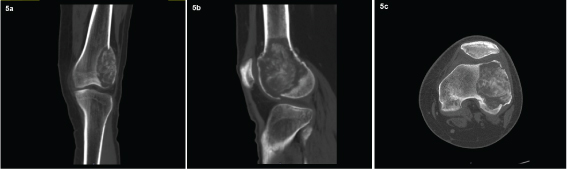
The patient subsequently received 4 doses of denosumab over a period of 2 months after checking serum calcium and phosphorous levels each time (120mg denosumab administered subcutaneously every 4weeks, with additional doses on day 8 and 15 of the first month of therapy). During treatment, the patient did not experience any adverse reactions. He was immobilized in a knee brace and was symptomatically better during this period. After the 4th dose of denosumab, patient was evaluated with imaging (MDCT left knee) which showed regression of lesion in size [Table/Fig-5a-c], and hence planned for extended bone curettage and sandwich grafting.
Post denosumab treatment: CT showing regression of lytic lesion with new bone formation in the coronal, sagittal and axial planes.
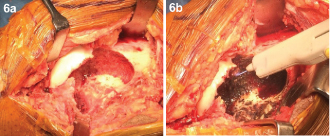
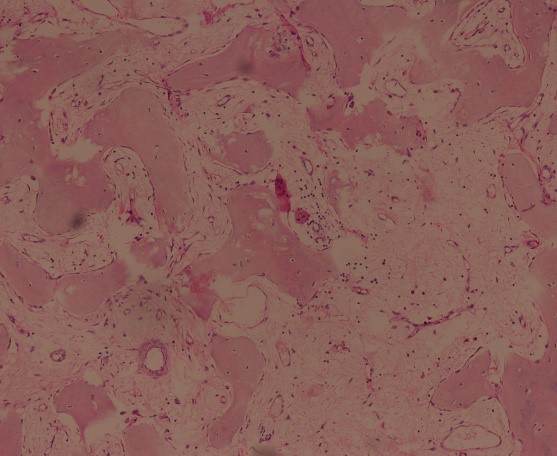
Extended curettage of the lesion was done with high speed burr and argon laser cautery followed by sandwich grafting with an autologous bone graft taken from iliac crest, gel foam, hydroxyappatite granules and bone cement [Table/Fig-6a&b]. Histopathological examination of the centre, margin and periphery of the tumour revealed an immature trabecular bone and osteoclast-type giant cells were not observed in the entire specimen in contrast to previous biopsy, indicating a good response to denosumab [Table/Fig-7].
(a) Intraoperative - Cavity after curettage. (b) Intraoperative- Using argon beam coagulation for extending curettage.
(H&E stain, 4X) Post curettage histopathology slides showing immature trabecular bone. Osteoclast-type giant cells were not observed.
Following surgery, the patient has continued to receive 120mg denosumab once per month for 5months. At the follow-up examination 15 months later, X-ray revealed good healing and knee flexion of 110 degrees. The patient currently has no pain and the present CT scan revealed no evidence of recurrence. The patient’s pre-operative clinical score according to the revised musculoskeletal tumour society scoring was 7/30 as compared to the postoperative clinical scoring of 29/30. At present, the patient does not suffer from any complaints and physical functioning is not impaired.
Discussion
The treatment goal of GCT is to remove the tumour completely and to preserve the affected joint and its function. As curettage alone was seen to be associated with high rate of local recurrence [1-4], various adjuvants and high-speed burr were used to extend the curettage [1,5]. The preferred treatment usually involves extensive curettage and packing the cavity with bone graft or cement [6]. GCTB is microscopically composed of multinucleated osteoclast like giant cells expressing RANK and mesenchymal spindle-like neoplastic stromal cells expressing RANKL (believed to play an important role in osteoclast formation, differentiation and survival). The interaction of these cells leads to resorption of bone [7].
Denosumab, a human monoclonal antibody, is a RANKL inhibitor and blocks its binding to RANK on osteoclasts and osteoclast precursors thus inhibiting the differentiation of osteoclasts and osteoclast-mediated bone resorption. Treatment of GCTB with denosumab has been reported to cause significant reduction in giant cells expressing RANK and stromal cells as well as replacement of these cells with new bone formation [8]. As a result, denosumab therapy may offer a promising option in the management of cases in which surgical treatment is associated with an increased morbidity [8,9]. In the present case, the patient had an excellent response to denosumab treatment prior to surgery. The post-curettage specimen, following denosumab therapy revealed the absence of osteoclast-type giant cells and presence of bone formation was observed when compared with the incisional biopsy.
Some of the issues that need to be addressed regarding denosumab treatment include the optimal duration of denosumab treatment is unknown and the toxicity profile raises concerns regarding long-term use of denosumab although certain patients may require it [10]. It has also been reported that recurrences have been observed following cessation of denosumab treatment [11].
Conclusion
Denosumab suppresses osteoclast differentiation thus reducing bone resorption and results in new bone formation. In the present case, denosumab demonstrated efficacy against giant cell tumour of the distal end of femur by causing complete tumour regression. Microscopic examination of post curettage specimen following denosumab treatment, revealed the absence of giant cells and the presence of newly formed immature trabecular bone.
[1]. Szendröi M, Giant-cell tumour of boneJ Bone Joint Surg Br 2004 86(1):5-12. [Google Scholar]
[2]. Algawahmed H, Turcotte R, Farrokhyar F, Ghert M, High-speed burring with and without the use of surgical adjuvants in the intralesional management of giant cell tumour of bone: a systematic review and meta-analysisHindawi Publishing Corporation Sarcoma 2010 2010(article ID:586090):5 [Google Scholar]
[3]. Karpik M, Giant cell tumour (tumour gigantocellularis, osteoclastoma)—epidemiology, diagnosis, treatmentOrtop Traumatol Rehabil 2010 12(3):207-15. [Google Scholar]
[4]. Yu XC, Xu M, Song RX, Fu ZH, Liu XP, Long-term outcome of giant cell tumours of bone around the knee treated by en bloc resection of tumour and reconstruction with prosthesisOrthop Surg 2010 2(3):211-17. [Google Scholar]
[5]. Turcotte RE, Giant cell tumour of boneOrthop Clin North Am 2006 37(1):35-51. [Google Scholar]
[6]. Puthoor D, Iype W, Giant cell tumour: Curettage and bone graftingIndian J Orthop 2007 41(2):121-23. [Google Scholar]
[7]. Bekker PJ, Holloway DL, Rasmussen AS, Murphy R, Martin SW, Leese PT, A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal womenJ Bone Miner Res 2004 19:1059-66. [Google Scholar]
[8]. Branstetter DG, Nelson SD, Manivel JC, Blay JY, Chawla S, Thomas DM, Denosumab induces tumour reduction and bone formation in patients with giant-cell tumour of boneClin Cancer Res 2012 18:4415-24. [Google Scholar]
[9]. Yamagishi T, Kawashima H, Ogose A, Sasaki T, Hotta T, Inagawa S, Disappearance of giant cells and presence of newly formed bone in the pulmonary metastasis of a sacral giant-cell tumour following denosumab treatment: A case reportOncol Lett 2016 11(1):243-46. [Google Scholar]
[10]. Mak IW, Evaniew N, Popovic S, Tozer R, Ghert M, A translational study of the neoplastic cells of giant cell tumour of bone following neoadjuvant denosumabJ Bone Joint Surg Am 2014 96(15):e127 [Google Scholar]
[11]. Skubitz KM, Giant cell tumour of bone: Current treatment optionsCurr Treat Options Oncol 2014 15:507-18. [Google Scholar]