Eosinophilic Granulomatosis with Polyangiitis (EGPA) or Churg-Strauss syndrome is a rare systemic illness which is characterized by necrotizing inflammation of small and medium sized vessels. The prominent features include asthma, eosinophilia, transient pulmonary infiltration and systemic vasculitis. Various triggering factors have been reported as putative aetiological agents of Churg-Strauss syndrome. They include infections, allergic desensitization, vaccinations like hepatitis B and influenza vaccination, cocaine abuse, drugs like leukotriene receptor antagonists and exposure to pigeons. We report a rare case of Churg–Strauss syndrome co-infected with Hepatitis B Virus (HBV). Very few such cases have been reported in the past.
Asthma, Eosinophilia, Necrotizing
Case Report
A 25-year-old male patient was admitted at a tertiary care hospital in the state of Uttrakhand with complaints of low grade fever and malaise for the past two months. He also complained of pain and paresthesia in left leg and foot since two months. He developed abdominal pain in right upper quadrant severe in intensity and stabbing in nature since 15 days. Pain in abdomen aggravated with food intake and was associated with vomiting which was non projectile in nature and was non blood stained. After five days of pain in abdomen he developed palpable purpuric rashes on both the legs. After 4 days of hospital stay his skin lesions worsened and ulcerated. These were well defined ulcers covered at places with haemorrhagic crusting and at places with purulent discharge [Table/Fig-1a-c]. Patient was a known case of bronchial asthma since the age of 15 years. He did not consume leukotriene receptor antagonists in the past. There was no history of cocaine abuse, hepatitis B or influenza vaccination and bird exposure.
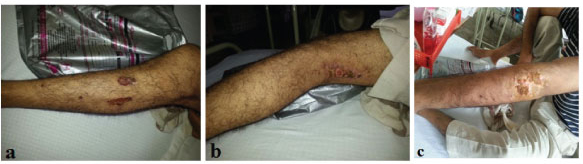
On general physical examination, patient was conscious and oriented, afebrile, blood pressure-120/80mmHg in right arm in supine position, pulse-88beats per minute, regular, all peripheral pulsations palpable, respiratory rate-22 breaths/minute, SpO2-100%, mild pallor was present, icterus, lymphadenopathy, clubbing and pedal oedema were absent. On respiratory system evaluation, bilateral wheeze was present.
Nervous system examination: Sensory system-decreased sensations over dorsal aspect of left foot in the area of distribution of common peroneal nerve; Motor system–power of bilateral lower and upper limbs was 5/5 except that of dorsiflexors of left foot whose power was 3/5.
All reflexes were preserved except left sided ankle jerk which was absent. Gait was observed to be a high stepping gait.
Per abdomen examination: Mild tenderness was present over right hypochondrium region with hepatomegaly + 2 cm below the right costal margin and spleen was just palpable.
Haematological investigations: Mild anaemia with haemoglobin level of 11g/dl, leukocytosis (total leukocyte count-24560/mm3) with 31% of eosinophils. He exhibited serum IgE levels of 800mg/dl. Parasitological studies were normal. Absolute eosinophil count was 25600/mm3.
Liver function tests: Mildly deranged [total bilirubin-1.38mg/dl, direct bilirubin-0.56mg/dl, Alanine Transaminase (ALT)-172IU/l, Aspartate Transaminase (AST) - 62IU/l, Alkaline Phosphatase (ALP) -129IU/l, albumin-2.37mg/dl, total protein-5mg/dl]. Prothrombin time of the patient was 18.3 seconds with INR of 1.53 which was mildly raised.
Viral markers: Hepatitis B surface antigen (HBsAg) was reactive whereas other viral markers and HIV were non reactive.
Urine routine and microscopy showed WBC 5-10/hpf, RBC 5-10/hpf and WBC cast were present. His stool for occult blood was positive but his upper gastrointestinal endoscopy was normal.
Radiological investigations: USG abdomen was suggestive of hepatomegaly with hypoechoic liver with splenomegaly with gall bladder sludge. Chest X-ray was normal. X-ray of paranasal sinuses was suggestive of bilateral maxillary sinusitis.
Nerve Conduction Velocity (NCV) was done which was suggestive of axonal sensory motor neuropathy. Since it was only restricted to distribution of common peroneal nerve involving both the lower limbs- a possibility of mononeuritis multiplex was kept.
Anti-Neutrophilic Antibody (ANA) and Anti Neutrophilic Cytoplasmic Antibody (ANCA) were negative.
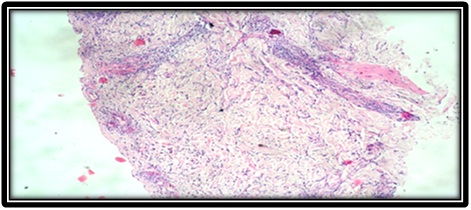
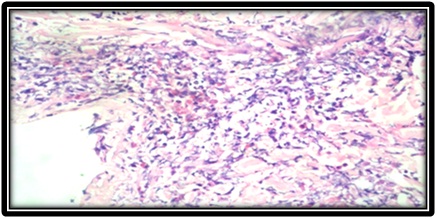
Skin biopsy showed thinned out and atrophic epidermis [Table/Fig-2]. Dermis showed numerous scattered small to medium sized blood vessels which were surrounded and infiltrated by neutrophils and fibrinoid necrosis [Table/Fig-3,4]. Few of the small vessels showed fibrin thrombi in the vessel walls. Epitheloid granuloma was not seen. With the help of above findings and clinical features diagnosis of Churg-Strauss syndrome was made.
Atrophic dermis and small vessels surrounded by inflammatory cells.
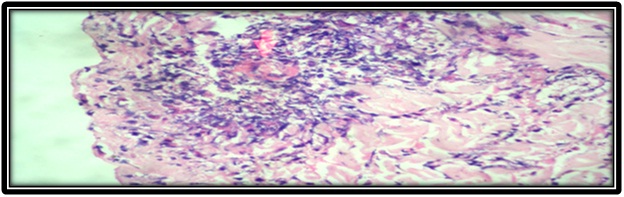
Eosinophilic infiltrates.
Management: Patient was started on oral steroid prednisone at the dose of 1mg/kg/day and was increased to 2mg/kg/day (100mg/day). As the response was slow azathioprine was added in the dose of 50mg twice a day. Patient had improvement in the skin lesions and on follow-up also had improvement in the foot drop.
Discussion
EGPA also called as Churg-Strauss syndrome is a rare form of systemic vasculitis, affecting less than two people in a million population being diagnosed each year.
People around the age of 50 years are most commonly affected although it has been reported even in younger age group. Our patient was 25 years of age. In 1990, the American College of Rheumatology (ACR) proposed the following six criteria for the diagnosis of Churg-Strauss syndrome: Asthma (wheezing, expiratory rhonchi), Eosinophilia of more than 10% in peripheral blood, Paranasal sinusitis, Pulmonary infiltrates (may be transient), histological proof of vasculitis with extravascular eosinophils, Mononeuritis multiplex or polyneuropathy. The presence of four or more criteria yields a sensitivity of 85% and a specificity of 99.7% [1]. Our patient was a known case of bronchial asthma. He had eosinophilia of more than 10% in peripheral blood, paranasal sinusitis, mononeuritis multiplex and evidence of vasculitis proved by histochemical findings. Hence, we made the diagnosis of Churg- Strauss syndrome or EGPA. The underlying pathophysiology of Churg-Strauss syndrome is an autoimmune process which is marked by allergic process, alteration in humeral pathway of immunity, increase in T-cell immunity and increase in immune complex deposition [2]. Clinically EGPA is divided in three phases. During the first phase called as allergic phase, patient has asthma, nasal polypi and allergic rhinitis. The second phase is termed as eosinophilic phase which is associated with chronic eosinophilic pneumonia, eosiniphilic gastroenteritis and eosinophilia in the blood. The third stage is that of vasculitis phase which shows multiorgan involvement like that of lung, heart, skin or CNS [3]. Churg-Strauss syndrome is closely linked to atopy and asthma. In a genetically susceptible patient, any foreign antigen can precipitate this allergic inflammation, thus causing rhinosinusitis, eosinophilic pneumonia or gastroenteritis. Vascular inflammation is followed by endothelial cell adhesion and activation of leucocytes, eventually causing necrotizing vasculitis of various organs like heart, lung, peripheral nervous system, skin and gastrointestinal system [4]. Vasculitic phase of the syndrome is associated with increased IgE levels [5]. ANCA antibodies lead to neutrophil activation and degranulation. ANCA is present in 48-66% of EGPA patients [6]. It is found that one third of EGPA patients don’t have ANCA in their serum. Thus, ANCA cannot be attributed as a cause of Churg-Strauss syndrome [7]. Our patient also did not have ANCA in his serum. Despite having good prognosis with steroids and other immunosuppressive agents, involvement of cardiovascular system, central nervous system and renal system is associated with poorer prognosis [4].
Although the aetiology of Churg-Strauss syndrome is not well understood, few case reports in the past have suggested that there are some foreign agents which trigger the inflammation cascade in these patients. Exposure to pigeons, allergic desensitization, cocaine abuse, use of leukotriene receptor inhibitors and hepatitis B vaccination have surfaced as common triggering agents in these patients [4,8–10]. Very few case reports have reported a rare association of EGPA with hepatitis B infection [11]. The first case report of co-existence of hepatitis B infection with Churg-Strauss syndrome was by Kin YB in year 2000 [12]. Our case also highlights this rare co-existence of both hepatitis B and Churg-Strauss syndrome.
Conclusion
This observation should be kept clinically in mind that hepatitis B infection can also act as a triggering agent of Churg-Strauss syndrome or EGPA.
[1]. Masi AT, Hunder GG, Lie JT, Michel BA, Bloch DA, Arend WP, The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis)Arthritis Rheum 1990 33:1094-100. [Google Scholar]
[2]. Fu MH, Tsai WC, Lan J, Lu CH, Lee LH, Huang CC, Churg-Strauss syndrome following vaccination against 2010 influenza A (H1N1)Acta Neurol Taiwan 2014 23:95-101. [Google Scholar]
[3]. Streho M, Sable-Fourtassou R, Brion MC, Bourotte I, Valeyre D, Brauner M, Churg-Strauss syndrome and pulmonary fibrosis: An unusual associationPresse Med 2006 35(pt 1):1259-62. [Google Scholar]
[4]. Noth I, Strek ME, Leff AR, Churg-Strauss syndromeLancet 2003 361:587-94. [Google Scholar]
[5]. Lanham JG, Elkon KB, Pusey CD, Hughes GR, Systemic vasculitis with asthma and eosinophilia: A clinical approach to the Churg-Strauss syndromeMedicine (Baltimore) 1984 63:65-81. [Google Scholar]
[6]. Hagen EC, Daha MR, Hermans J, Andrassy K, Csernok E, Gaskin G, Diagnostic value of standardized assays for anti-neutrophil cytoplasmic antibodies in idiopathic systemic vasculitisKidney Int 1998 53:743-53. [Google Scholar]
[7]. Jennette JC, Falk RJ, Andrassy K, Bacon PA, Churg J, Gross WL, Nomenclature of systemic vasculitides: Proposal of an international consensus conferenceArthritis Rheum 1994 37:187-92. [Google Scholar]
[8]. Guillevin L, Amouroux J, Arbeille B, Boura R, Churg-Strauss angitis: Arguments favouring the responsibility of inhaled antigensChest 1991 100:1472-73. [Google Scholar]
[9]. Orriols R, Muñoz X, Ferrer J, Huget P, Morell F, Cocaine-induced Churg-Strauss vasculitisEur Respir J 1996 9:175-77. [Google Scholar]
[10]. Vanoli M, Gambini D, Scorza R, A case of Churg-Strauss vasculitis after hepatitis B vaccinationAnn Rheum Dis 1998 57:256-57. [Google Scholar]
[11]. Domiciano DS, Shinjo SK, Neto ML, Churg-Strauss Syndrome and Active Chronic Hepatitis B Virus Infection: Coincidence or Association?Clinics (Sao Paulo) 2010 65(3):335-36. [Google Scholar]
[12]. Kim YB, Choi SW, Park IS, Han JY, Hur YS, Chu YC, Churg-Strauss syndrome with perforating ulcers of the colonJ Korean Med Sci 2000 15:585-88. [Google Scholar]