World Health Organization (WHO) defines Fixed Dose Combination (FDC) as “A combination of two or more active ingredients in a fixed ratio of doses and in a single dosage form” [1]. FDC products are acceptable when it has a proven advantage over single compounds administered separately in therapeutic effect, safety or compliance and when the dosage of each ingredient meets the requirement of a defined population group [2]. WHO Essential Medicine List (EML) 2015 includes 27 FDCs which are safe, effective and used for the treatment of Human Immunodeficiency Virus (HIV), tuberculosis and contraception etc [3]. FDCs are considered as new drugs and must comply the Schedule Y for marketing approval in India. The Drug Controller General of India (DCGI) approved 1,125 FDC formulations between 1961 and 2013 [4]. Multiple deficiencies in approval process of the DCGI were highlighted by Department related standing committee on Health and Family Welfare report in 2012 [5]. The proliferation of FDCs in Indian market has raised several questions on their safety, rationality and justification [4]. Moreover, several FDCs available in Indian market are rejected by regulators in Europe, North America, and Australia [5].
FDCs have been categorized into four types for regulatory approval. Type one includes one or more new, previously not approved Active Pharmaceutical Ingredients (APIs) and the regulatory requirement to manufacture and market these FDCs, same as any other new drug. Type two categories consist of already approved or marketed APIs as single ingredient. However, these APIs are combined for the first time and likely to have significant pharmacokinetic or dynamic advantage. Moreover, a summary of all necessary pharmacological, toxicological and clinical data of individual drug along with the rationale of combining them is necessary for the approval of FDC. While the third type of FDCs are also previously marketed in India but there is either change in the ratio of API or proposed for a new therapeutic use or a new dosage form. Interestingly, the fourth type of FDCs includes widely and concomitantly used individual APIs for a particular indication for years and no claim (kinetic or dynamic) is proposed other than ‘convenience’ for combining the APIs. In addition, the fourth types of FDCs do not require animal or human data [6]. Thus, the Indian regulatory requirements for FDCs are different and loosely match with WHO guidelines [1]. Further, the fourth category FDCs are based on ‘convenience’ and has no comparable in WHO classification. Considering a large number of FDCs for Cardiovascular (CV) and Central Nervous System (CNS) diseases available in Indian market, an attempt has been made to investigate their rationality in these two therapeutic areas.
Materials and Methods
A cross-sectional, observational study was conducted at the Department of Pharmacology, B. J. Medical College, Ahmedabad, Gujarat. The data was collected from an annual Drug Compendium entitled “Indian Drug Review” (IDR) 2014, Issue 3 that enlists most of the medicines commercially available in India during a particular year [7,8]. FDCs enlisted in CV section and CNS section were assessed. The rationality of FDCs was assessed by a pre-tested, validated tool designed by Shah et al., based on WHO guidelines [9] [Table/Fig-1]. Standard textbooks and reference books of pharmacology and medicine along with authentic web sources like PubMed data base, Google scholar and Cochrane data base were searched for the evidence of efficacy and safety of the individual API and their combination. The total score ranged from 1 to 12 and if the score is ≥7, FDC was considered rational [9]. The data was entered in Microsoft excel sheet and analysed.
Tool to assess the rationality of Fixed Dose Combinations available in Indian market.
| 1. Active pharmacological ingredient along with strength2. API |
| API |
| 1 Approved by DCGI | Yes (+1) | No (−1) |
| 2 Ingredient: Banned or Controversial | Yes (−1) | No (+1) |
| 3. Listing in EML | WHO/National/Both/None |
| (+1) | (0) |
| 4. Efficacy (text book/reference book/pub med/medline/other) |
| 1 API | Yes (+1) | No (0) |
| 2 FDC | Yes (+1) | No (0) |
| 5. Safety (text book/reference book/pub med/medline/other) |
| 1 API | Yes (+1) | No (0) |
| 2 FDC | Yes (+1) | No (0) |
| 6. Pharmacokinetic (absorption/distribution/metabolism/excretion/BA/BE/t ½)• Interaction Favourable/Unfavourable/Not affected (+1) (−1) (0) |
| 7. Pharmacodynamic – | M /A of each ingredientSimilar (0) / Different (+1) |
| 8. Advantage of FDC• Reduced• Less ADR• Convenient (frequency or pill count) | Yes (+1) / No (0)Yes (+1) / No (0)Yes (+1) / No (0) |
| Total score: 12Score ≥7: Rational FDCScore ≤6: Irrational FDC |
API = Active pharmacological ingredient, DCGI = Drug Controller General of India, FDC = Fixed dose combination
For analysis, FDCs were categorized into four groups. Group A comprised of DCGI approved plus rational and group B was DCGI approved plus irrational. While group C included DCGI unapproved plus rational and group D was DCGI unapproved plus irrational. The list of FDCs approved by DCGI was procured from CDSCO website. (www.cdsco.nic.in). The data were analysed using ANOVA followed by post-hoc analysis by Tukey test. The p < 0.05 was considered as statistical significant.
Results
Out of 152 FDCs, 107 belonged to cardiovascular (CV) group and 45 to CNS and 77 were approved and 75 were unapproved by Central Drugs Standard Control Organization.
Details of Active Pharmacological Ingredients
Majority of FDCs (80, CV and 33, CNS) contained two APIs [Table/Fig-2]. Secondly, 132 FDCs contained approved API while 16 had one or more unapproved API by DCGI. However, no FDC was found to be banned or declared controversial by DCGI.
Assessment of Cardiovascular and Central Nervous Ssystem FDCs using rationality tool (n = 152).
| Parameters | CVS FDCs | CNS FDCs (n=45) |
|---|
| DCGI approved | 64 | 13 |
| Mean rationality score (CI - 95 %) | 6.72±2.82 (3.90 - 9.54) | 6.22±2.08 (4.14 - 8.30) |
| Number of rational FDCs | 46 | 8 |
| Number of irrational FDCs | 61 | 37 |
| Number of API in each FDC |
| 2 | 80 | 33 |
| 3 | 16 | 7 |
| ≥4 | 11 | 5 |
CVS = Cardiovascular system, CNS = Central nervous system, FDC = Fixed dose combination, API = Active pharmacological ingredients, DCGI = Drug Controller General of India, CI = Confidence interval
The most common rational CV FDCs were combination of beta blocker plus thiazide diuretic (7), Angiotensin Receptor Blocker (ARB) plus thiazide diuretic (7), Angiotensin Converting Enzyme Inhibitor (ACEI) plus thiazide diuretic (5) [Table/Fig-3]. Surprisingly, certain CV FDCs (11) contained controversial ingredients such as lycopene, rutin, policosanol, adrenochrome monosemicarbazone, methylhesperidine, co enzyme Q10, l carnitine l tartrate, grape seed extract. While the rational CNS FDCs were combinations of opioid agonist/antagonist plus NSAIDs (3), dopamine precursors plus dopa decarboxylase inhibitor (2) [Table/Fig-3].
Rational Cardiovascular and Central Nervous System fixed dose combinations.
| Cardiovascular FDCs (n=46) |
|---|
| Beta blocker plus Thiazide diuretic (7)Bisoprolol / Metoprolol / Nebivolol / Atenolol / S-Atenolol + HydrochlorothiazideAtenolol + Indapamide / Chlorthalidone | Potassium sparing diuretic plus Thiazide diuretic (3)Amiloride + HydrochlorothiazideSpironolactone + HydroflumethiazideTriamterene + Benzthiazide |
| ARB plus Thiazide diuretic (7)Candesartan / Irbesartan / Losartan / Olmesartan / Telmisartan / Valsartan + HydrochlorothiazideTelmisartan + Chlorthalidone | CCB plus ARB (3)Amlodipine + Olmesartan / Losartan / Telmisartan |
| ACEI plus Thiazide diuretic (5)Captopril / Lisinopril / Enalapril / Ramipril + HydrochlorothiazidePerindopril + Indapamide | ARB plus CCB plus Thiazide (2)Olmesartan / Losartan + Amlodipine + Hydrochlorothiazide |
| Statins plus Other lipid lowering agents (5)Atorvastatin + Nicotinic acidAtorvastatin / Simvastatin + EzetimibeAtorvastatin / Rosuvastatin + Fenofibrate | CCB plus Beta blocker (2)Amlodipine + MetoprololAtenolol + Nifedipine |
| CCB plus ACEI (4)Amlodipine + Ramipril / Benazepril / Enalapril / Perindopril | Antiplatelet plus Antiplatelet (2)Aspirin + Clopidogrel / Dipyridamole |
| High ceiling diuretic plus Potassium sparing diuretic (4)Furosemide + Triamterene / Amiloride / SpironolactoneTorasemide + Spironolactone | ARB plus Beta blocker (1)Nebivolol + Valsartan |
| CCB plus Beta blocker plus Thiazide (1)Amlodipine + Atenolol + Hydrochlorothiazide |
| Central Nervous System (n=8) |
| Opioid agonist / antagonist plus NSAIDs (3)Codeine / Pentazocine / Tramadol + Paracetamol | Anti-migraine (2)Domperidone / Metoclopramide + Paracetamol |
| Dopamine precursors plus Dopa decarboxylase inhibitor (2)Levodopa + Carbidopa* / Benserazide | Benzodiazepine plus Hypnotic (1)Alprazolam + Melatonin |
CCB - Calcium channel blocker, ACEI - Angiotensin converting enzyme inhibitor, ARB - Angiotensin receptor blocker, NSAIDs - Non steroidal anti-inflammatory drugs, * FDC included in WHO EML 2015
Listing in Essential Medicine List
Surprisingly, only one FDC having combination of levodopa plus carbidopa was listed in both WHO EML 2015 and National List of Essential Medicines of India, 2015 [Table/Fig-3].
Evidence of Efficacy and Safety of API & FDC
It was observed that out of 152 FDCs, efficacy and safety of 40 was well documented and proved in clinical trials or meta analysis. While 11 FDCs contain API with controversial efficacy.
Assessment of Pharmacokinetic and Pharmacodynamic interaction
Favorable Pharmacokinetic interaction was observed in only three FDCs (levodopa plus carbidopa, levodopa plus benserazide and metoclopramide plus paracetamol) while unfavorable pharmacokinetic interaction was found in five FDCs. Moreover, no interaction between APIs was found in 144 FDCs. Further, majority of FDCs (147), API had different mechanisms of action. However, in five FDCs, API had similar mechanism of action.
Advantage(s) of FDC
Out of 152 FDCs, 32 showed advantage of reducing Adverse Drug Reactions (ADRs) as compared to individual ingredient [Table/Fig-4]. To a great surprise, all FDCs showed advantage of being convenient by reducing pill count or frequency of administration. Moreover, none of the FDCs showed advantage of dose reduction of individual active ingredient.
List of rational FDCs that reduce the Aadverse Drug Reaction (ADR) (32).
| ARB plus Thiazide diuretic (7)Candesartan / Irbesartan / Losartan / Olmesartan / Telmisartan / Valsartan + HydrochlorothiazideTelmisartan + Chlorthalidone | Potassium sparing diuretic plus Thiazide diuretic (3)Amiloride + HydrochlorothiazideSpironolactone + HydroflumethiazideTriamterene + Benzthiazide |
| Beta blocker plus Thiazide diuretic (7)Atenolol + Indapamide / Hydrochlorothiazide / ChlorthalidoneBisoprolol / Metoprolol / Nebivolol / S-atenolol + Hydrochlorothiazide | ARB plus CCB plus Thiazide diuretic (2)Olmesartan / Losartan + Amlodipine + Hydrochlorothiazide |
| ACEI plus Thiazide diuretic (5)Captopril / Enalapril / Lisinopril / Ramipril + HydrochlorothiazidePerindopril + Indapamide | CCB plus ACEI (1)Amlodipine + Enalapril |
| Potassium sparing diuretic plus High ceiling diuretic (4)Triamterene / Amiloride + FurosemideSpironolactone + Furosemide / Torasemide | CCB plus ARB (1)Amlodipine + Telmisartan |
| CCB plus Beta blocker (1)Nifedipine + Atenolol |
| CCB plus Beta blocker plus Thiazide diuretic (1)Amlodipine + Atenolol + Hydrochlorothiazide |
CCB - Calcium channel blocker, ACEI - Angiotensin converting enzyme inhibitor, ARB - Angiotensin receptor blocker
Assessment of Rationality
Out of 107 CV FDCs, 46 were rational and 61 were irrational with a mean rationality score of 6.72±2.82 (CI– 95%, 3.90-9.54). While out of 45 CNS FDCs, 8 were rational and 37 were irrational with a mean rationality score of 6.22±2.08 (CI – 95%, 4.14-8.30) [Table/Fig-2].
A significant difference in mean rationality score of Group A FDCs (DCGI approved + rational) was observed as compared to Group B FDCs (DCGI approved + irrational) (p< 0.05) [Table/Fig-5,6 and 7]. Similarly a significant difference in mean rationality score of Group C FDCs (DCGI unapproved + rational) was observed as compared to group D FDCs (DCGI unapproved + irrational) (p<0.05) [Table/Fig-7].
Analysis of groups by ANOVA.
| Source of variation | Degrees of freedom (df) | Sum of squares | Mean square | p |
|---|
| Between groups | 3 | 213.43 | 71.14 | < 0.0001 |
| Within Groups | 148 | 59.62 | 0.40 | |
| Total | 151 | 273.05 | | |
Post hoc analysis of groups by Tukey Krammer multiple comparison test.
| Comparison | Mean difference | Q value | p-value |
|---|
| Group A vs group B | 2.400 | 23.416 | p < 0.001 |
| Group A vs group C | 0.7071 | 4.912 | p < 0.01 |
| Group A vs group D | 2.720 | 30.111 | p < 0.001 |
| Group B vs group C | -1.699 | 11.697 | p < 0.001 |
| Group B vs group D | 0.3199 | 3.402 | p > 0.05 (ns) |
| Group C vs group D | 2.019 | 14.744 | p < 0.001 |
ns – non significant
Comparison of mean rationality score of FDCs as per DCGI approval.
*p< 0.001 as compared to DCGI approved plus rational
**p<0.001 as compared to DCGI unapproved plus rational
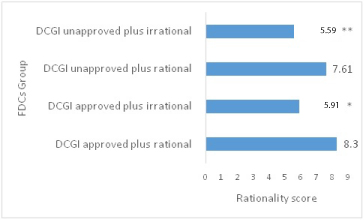
Among DCGI approved FDCs, 41 (53.24%) were rational and 36 (46.75%) were irrational with a mean rationality score of 7.22±2.68 (CI - 95%, 4.54 - 9.9), while among DCGI unapproved FDCs 13 (17.33%) were rational and 62 (82.66%) were irrational with a mean rationality score of 5.94±2.7 (CI - 95%, 3.24 - 8.64).
Discussion
The present study showed that a substantial numbers of CV and CNS FDCs are available in Indian market. Although the Drug regulators of India prevent the manufacture, distribution, sale of irrational FDCs, a lack of co-ordination with state licensing authorities and system to critically analyse the scientific validity has resulted into mushrooming of these formulations.
The study showed that, majority of these FDCs was irrational and not approved by DCGI. While API used in the majority of these formulations are individually approved by central regulatory authority, few FDCs contained unapproved API and found to be irrational. Surprisingly, only seven FDCs (phenytoin plus phenobarbitone, imipramine plus diazepam, paracetamol plus promethazine, chlorpromazine plus trihexyphenidyl etc.,) were included in banned drug list [10]. Availability of these FDCs raises safety concern and is important from a public health perspective.
Further, evidence of efficacy and safety of individual API was found, however, the scientific validity of majority of CV and CNS FDCs was lacking. The CVS FDCs with established efficacy and safety were combinations of thiazide diuretic plus beta blocker or ARB or ACEI and statins plus lipid lowering agents (statin plus nicotinic acid/ ezetimibe/ fibrate), antiplatelet FDCs [11-13]. Similarly the rational CNS FDCs were combination of opioid agonist/antagonist plus NSAIDs, dopamine precursors plus dopa decarboxylase inhibitor, anti-migraine FDCs, benzodiazepine plus hypnotic [14,15]. The combination of levodopa plus carbidopa increases efficacy due to pharmacokinetic advantage. The addition of carbidopa decreases the peripheral metabolism of levodopa, thereby, increasing its availability [16]. While the FDCs of metoclopramide plus paracetamol was found to have kinetc advantage. Metoclopramide being prokinetic agent increases the absorption of paracetamol from gut [17].
Besides efficacy, few of these rational FDCs also decrease the ADRs compared to individual API. These were combination of ARB or ACEI plus thiazide diuretic, ARB/ ACEI plus CCB, potassium sparing diuretic plus thiazide, high ceiling diuretic plus potassium sparing diuretic [18-24].
To a great surprise, the study showed FDCs with dubious ingredients which may be hazardous to human health. This indicates a prompt and strict regulatory action to safe guard consumers and to educate prescribers is necessary. Moreover, one of the most distinct observations was combination of these formulations was based on ‘convenience’ as these individual agents are concomitantly used in patients with cardiovascular disease such as atorvastatin plus aspirin, atorvastatin, ramipril plus clopidogrel / aspirin etc. The introduction of convenience (for patient) as a criterion in the Indian guidelines for FDC approval has deserted other consideration to potentially justify approval. Thus, it seems that type four ‘convenience’ category has been extensively exploited by the manufacturers, resulting into proliferation of FDCs and unnecessary exposure of drugs that may increase risk of adverse reactions and cause significant economic impact on patients.
The rationality tool also found that DCGI approved FDCs contain both rational and irrational FDCs. It can be suggested that irrational formulations can be banned or subjected for further efficacy and safety data. Interestingly, the rationality tool also showed a small number of FDCs which were DCGI unapproved but rational, for example, s-atenolol plus hydrochlorothiazide, telmisartan plus chlorthalidone, furosemide plus triamterene, paracetamol plus codeine etc. Due to scientific validity these FDCs may be considered for approval by central authorities. In addition, the assessment of rationality revealed that the mean rationality score was seven and more in rational FDCs irrespective of approval by central regulatory authority. Thus, this further validates the rationality tool and it can be used by prospective researchers and regulatory body.
The study findings confirm potentially harmful, unapproved and irrational FDCs in Indian market. Although the data was obtained from a commercial source, annual drug compendium and it is the most commonly used source of drug formulations information among the prescribers in India. A possibility of lack of complete information cannot be ruled out. Moreover, the study did not match the data with commercial sales record and the extent of use by the prescribers and patients. However, the data analysed leads to some important conclusions.
Conclusion
Thus, it can be concluded that the absence of watertight prerequisite and absence of critical analysis of the scientific validity of the formulations has resulted into a bizarre combinations and miserable scenario in the country. Although, FDCs have benefited patients in terms of efficacy and safety, the approval process needs to be rigorous to curb irrational combinations.
API = Active pharmacological ingredient, DCGI = Drug Controller General of India, FDC = Fixed dose combination