Diphtheroids are defined as aerobic, non-sporulating, pleomorphic Gram-positive bacilli which are more uniformly stained, lack the metachromatic granules and are arranged in a palisade manner. Although they are usually commensals of the skin and mucous membranes they are frequently reported in association with nosocomial infections and a vast majority of them are exhibiting antibiotic resistance [1–4]. Studies have reported multidrug resistant C. striatum, which was otherwise considered as a saprophyte of skin and mucous membrane to be a cause of long standing open wound infections and more recently as a cause of septic arthritis of a native knee joint and shoulder joint [5–7]. Likewise C. pseudotuberculosis has been shown to cause lymphadenitis in humans and because of the potential to cause zoonotic infections, milk and meat consumers are exposed to greater risk [1]. C. pseudodiphtheriticum has been mostly found to cause respiratory tract infections, nosocomial pneumonia, tracheitis, bronchitis and a number of other infections [8,9], C.minutissimun has been isolated from superficial skin infections and very rarely from invasive infections [10,11], C. ulcerans toxigenic strains have been isolated from nasopharyngeal infections; C. xerosis species have been found in conjunctival sac and on skin and mucous membranes [12]. The present study was conducted to identify the various species of diphtheroids isolated as pure growth from clinical specimens whose Gram’s smear revealed numerous inflammatory cells with Gram positive bacilli and had clinical evidence.
Diphtheroids isolated from bloodstream infections were considered clinically significant if clinical condition favoured infection and if there was pure growth of diphtheroids (within 48 hour).
The study also aimed at finding the antibiotic sensitivity pattern of various diphtheroid species and determining the ability of various diphtheroid species to form biofilms.
Materials and Methods
This study was carried out in the Department of Microbiology, Kasturba Medical College, Mangalore. Institutional ethical committee clearance was obtained for this study. It was an in-vitro cross- sectional study carried out for one year from Dec 2013 to Dec 2014. Corynebacterium spp isolated from pus, wound infections, blood, suction tip, catheter tip, ear swabs, sputum were used for the study. This comprised of a total of 100 isolates out of 16,242 various specimens received in the laboratory mainly from inpatients of the hospital. Specimens included sputum and endotracheal suction tips from patients with lower respiratory tract infection, ventilator associated pneumonia, ear swabs from chronic suppurative otitis media, catheter tips and blood from catheter associated bloodstream infections. Other specimens included pus from various lesions, wound swabs from diabetic foot ulcers, surgical wound infections and high vaginal swabs from patients with discharge.
The following criteria were considered for reporting of specimens:
In case of sputum, swabs and pus: Presence of pus cells and Gram positive bacilli in the smear and the culture yielding heavy growth of only Gram positive bacilli (diphtheroids) [1].
In case of IV catheter tips: Direct smear showing the presence of pus cells and pure growth of >15 colonies of Gram positive bacilli (diphtheroids) by semiquantitative culture technique of Maki et al., [13].
In case of endotracheal secretions: Direct smear showing the presence of pus cells and pure growth of >105cfu/mL of Gram positive bacilli (diphtheroids) by quantitative culture [14].
In case of blood culture: Presence of Gram positive bacilli in the direct smear from culture bottle taken after the indication of growth by the BacT-Alert system and BACTEC TM 9050 system, isolation of the same as pure growth, along with comparison with the graph shown by the instrument and correlation with clinical condition [15]
Microscopic examination included Gram’s staining of the specimen. Gram positive bacilli which were seen in the smear along with pus cells and isolated as pure growth were reported as “Corynebacterium spp having clinical significance”. These isolates were taken for this study while those which were reported as ‘Probable skin contaminants’ were excluded from this study. Species identification of Gram positive bacilli was done by catalase test, motility, OF test, nitrate reduction test, sugar fermentation test (glucose, sucrose, maltose, xylose, mannitol), urease test and esculin hydrolysis test [16]. Antibiotic susceptibility test was performed by Kirby-Bauer disk diffusion method. Antibiotics tested were Ampicillin (10mcg/disc), Cefaperazone–Sulbactam (75/30 mcg/disc), Chloramphenicol (30mcg/disc), Clindamycin (2mcg/disc), Ciproflaxacin (5mcg/disc), Erythromycin (10mcg/disc), Gentamycin (10mcg/disc), Imipenem (10mcg/disc), Linezolid (30mcg/disc), Nitrofurantoin (300mcg/disc), Oxacillin (1mcg/disc), Penicillin (10mcg/disc), Tetracycline (30mcg/disc) and Vancomycin (30mcg/disc). Since CLSI guidelines for disc diffusion method for diphtheroids is lacking, we followed the method used by Reddy BS et al., [16] which had adopted the British Society for Antimicrobial Chemotherapy (BSAC) guidelines for Ciprofloxacin, Penicillin and Vancomycin [17] while for the other antibiotics CLSI 2014 guidelines for Staphylococcus aureus were followed. S.aureus ATCC 25923 was used as control [18]. Biofilm production was done on all isolates by the microtitre plate method of O’Toole and Kolter [19–21]. The organisms were grown in brain heart infusion broth for 24 hour. Cultures were diluted 1:100 with fresh brain heart infusion broth and 200μl was inoculated into flat bottomed 96 well tissue culture plates and incubated at suitable temperature (37°C) separately for 48 hour. The contents of each well was gently aspirated by tapping the plate and placing micropipette tip in the lowest corner of the well. Using the micropipette, the wells were washed with 200μl Phosphate Buffered Saline (PBS pH-7.2). Adherent organisms were fixed in place with Bouin fixative and stained with Hucker crystal violet. Then the plate was washed with water. After drying, Optical Density (OD) of stained adherent bacterial films was read with micro ELISA plate reader and OD 570 was spectrophotometrically recorded.
Statistical analysis was done by using proportion test and Chi-square test. The confidence level of the test was 95% and power of the test was 80%.
Results
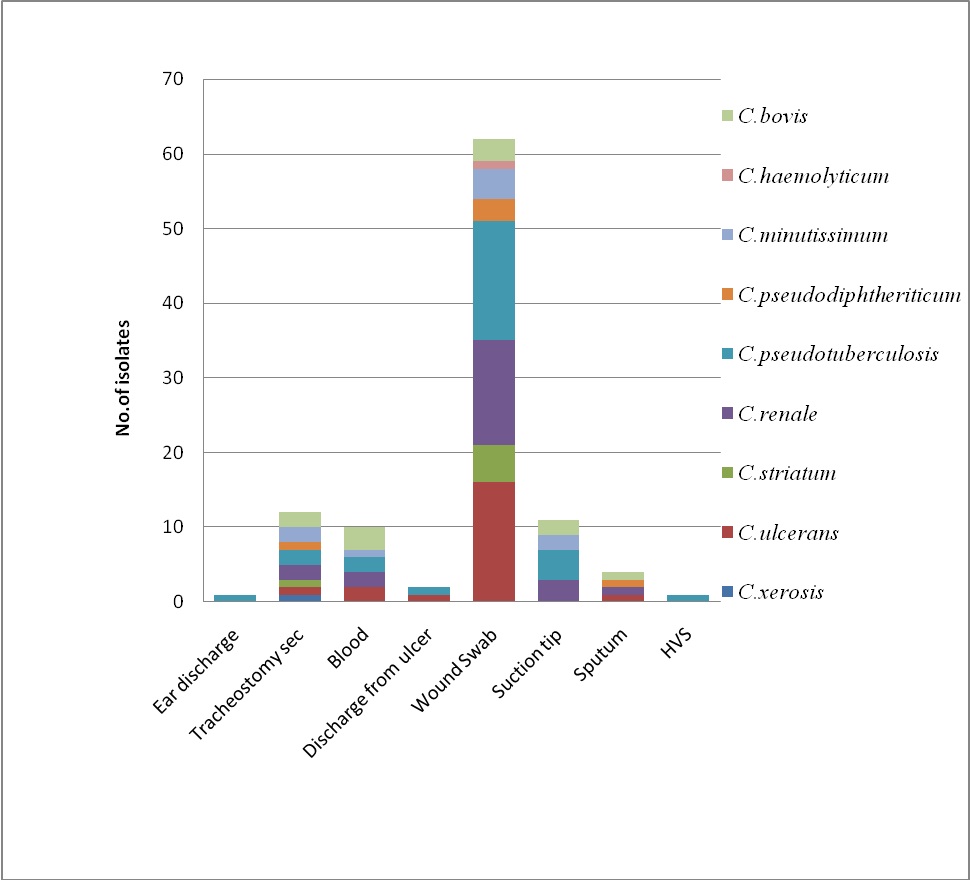
Among the Corynebacterium spps. isolated from pus, 5 out of 19 (26.31%) were C. pseudotuberculosis while C. renale was 21.05% followed by C. bovis 15.78%, C. ulcerans, C. minutissimum and C. pseudodiphtheriticum 10.52% [Table/Fig-1]. While 14 out of 43 isolates (32.5%) from wound infections were C. ulcerans, the others were C. pseudotuberculosis and C. renale (23.25%), C. striatum (9.30%), C. minutissimum (4.65%), C. haemolyticum (2.32%) and C. pseudodiphtheriticum (2.32%). Hence, the highest number of isolates was from wound infections and maximum were Coryebacterium pseudotuberculosis (26%).
Isolation of Corynebacterium spp. from various clinical specimens.
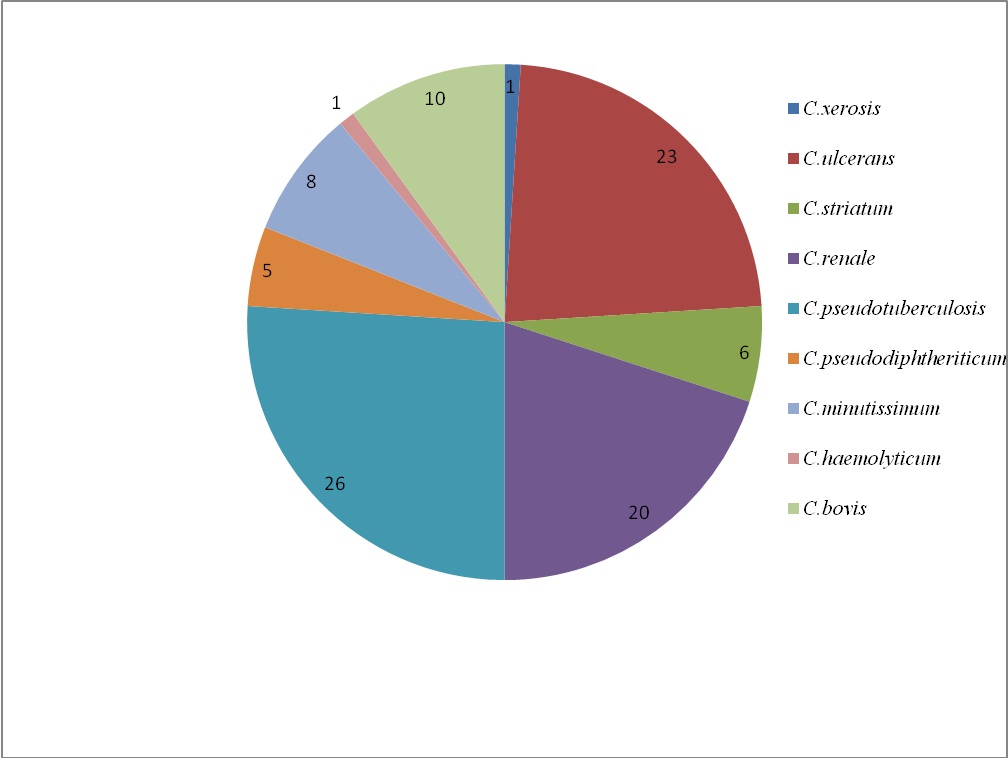
Out of 100 isolates 26% were Corynebacterium pseudotuberculosis, 23% Corynebacterium ulcerans, 20% Corynebacterium renale, 10% Corynebacterium bovis and rest of them were Corynebacterium striatum, Corynebacterium minutissimum, Corynebacterium pseudodiphtheriticum and Corynebacterium haemolyticum [Table/Fig-2].
Percentage isolation of various Corynebacterium spp.
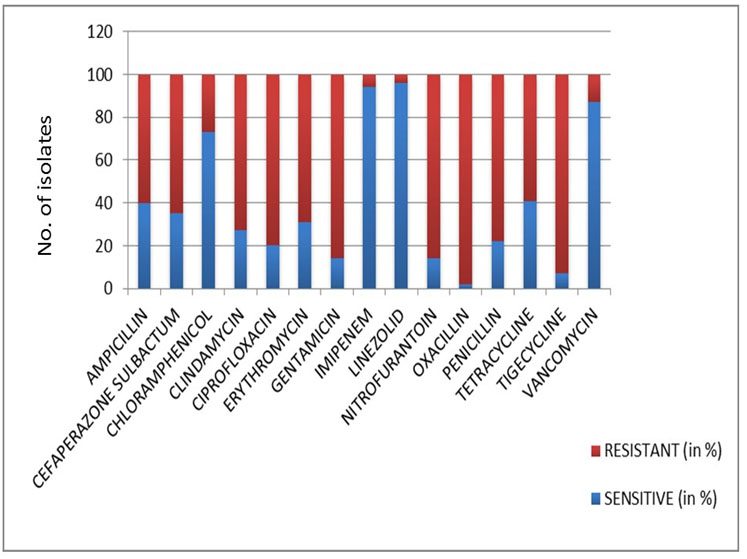
C. pseudotuberculosis, C. renale, C. ulcerans, C. striatum, C. minu-tissimum, Corynebacterium haemolyticum isolated from catheter tips, sputum, tracheostomy secretions and wound infections were highly resistant to Ampicillin, Gentamicin, Cefaperazone sulbactam, Ciprofloxacin, Clindamycin, Erythromycin, nitrofurantoin, oxacillin, penicillin and tetracycline, while isolates from blood namely C. pseudotuberculosis, C. minutissimum, C. ulcerans and C. renale were nearly sensitive to most of the antimicrobial agents. Almost all the isolates were sensitive to Vancomycin, Imipinem, Linezolid and Chloramphenicol [Table/Fig-3].
Antibiotic sensitivity pattern of the isolates.
With regard to the production of biofilm, there was an increased rate of biofilm in organisms isolated from wound infections, (Mean OD570 of 0.512), followed by catheter tip isolates (Mean OD570 of 0.465) and isolates from tracheostomy secretion (Mean OD570 of 0.300) [Table/Fig-4].
Absorbance values (OD570) of biofilms produced by isolates from different specimens.
| Specimens | OD570 |
|---|
| Swabs and pus from Wound infection | 0.512 |
| Catheter tip from bloodstream infection | 0.465 |
| Tracheostomy secretion from ventilator associated pneumonia | 0.300 |
| Sputum from respiratory tract infection | 0.280 |
| Blood culture isolates | 0.275 |
| Swab from Ear discharge | 0.200 |
| Discharge from ulcer | 0.130 |
| High Vaginal Swab | 0.090 |
Discussion
In routine diagnostic practice diphtheroids are commonly considered as contaminants from the skin [1–3]. They are usually not identified to the species level and antimicrobial susceptibility testing is not performed. Certain studies report antimicrobial resistance in Corynebacterium species such C. amycolatum, C. jeikeium, C. minutissimum, C. pseudodiphtheriticum, C. resistens, C. striatum, C. tuberculostearicum, C. urealyticum [22–24]. Otherwise, multidrug resistance among the infrequently recovered Corynebacterium species is rarely observed. One study suggested that C. jeikeium and C. urealyticum are more susceptible to teicoplanin and vancomycin as well as to the tetracyclines [21] whereas in our study, the isolates were resistant to tetracyclines. Diphtheroids isolated from blood cultures however were sensitive to most of the antibiotics while the others exhibited a high degree of resistance, which could in turn complicate patient management. This was an interesting finding and this could probably be due to the fact that diptheroids being less invasive may have been less exposed to antibiotics in the bloodstream. Resistance to linezolid and vancomycin is hardly encountered but the drawback is the restricted use of vancomycin due to its potential nephrotoxicity. Some earlier study has shown that most of the diphtheroids were susceptible to Tigecycline thus suggesting that it could be used for empirical treatment [25]. However, in the present study a high level of resistance to Tigecycline was exhibited by the isolates. Previous studies have highlighted the ability of isolates from catheter and prostatic infections to produce biofilm and thereby to recurrent infections thus making the biofilm resistant to antibiotics [26–28]. It is very interesting to note that biofilm production was maximum in isolates from catheter tips, tracheostomy secretions, sputum, wound infections and as mentioned earlier they were the ones that showed multidrug resistance. The biofilm nature and structure are responsible for resistance to antibiotics and it has been explained by any of the possible mechanisms namely delayed penetration of the antibiotic through the biofilm matrix, altered growth rate of organisms in the biofilm and the physiological changes occuring in the organisms due to the biofilm mode of growth [29].
Limitation
The major limitation of our study was the lack of detection of Minimum Inhibitory Concentration (MIC) of antibiotic as well as the study of the genotype responsible for biofilm production.
Conclusion
The increasing multidrug resistance of diphtheroids warrants the need for antimicrobial susceptibility testing of these organisms and also device ways of preventing infection with these skin colonizers. The high level of multidrug resistance exhibited by catheter tip isolates also shows that these organisms are predominantly nosocomial pathogens. Hence, when one considers the prevention of healthcare associated infections, it is very important to consider the risk of acquiring infection through contact of the hospital personnel who may be colonized with multidrug resistant diphtheroids. Nevertheless, diphtheroids can also cause endogenous infections and is very important in case of patients who have undergone various procedures and are in the intensive care units. The major risk factor associated with these organisms as depicted by our study is the production of biofilms. Hence, we can arrive at the conclusion that the nosocomial strains of diphtheroids survive in the form of biofilms and cause multidrug resistant infections. If they are initially considered as mere skin contaminants, there is a greater risk that they may acquire more and more of resistance. Since, they are usually present as skin colonizers, one needs to judge the isolation of these organisms, identify them using newer techniques like Mass spectrometric identification and perform the antimicrobial susceptibility to correlate with the clinical significance. Besides, practices to avoid the spread of hospital associated infections should be followed stringently bearing in mind the role of diphtheroids as potential nosocomial pathogens. Thus, the take home message of this study is that these organisms cannot be ignored but need to be given due consideration depending on the clinical condition.