Introduction
There is an increase in oral cancer burden day by day. The mainstream treatment modalities in treating cancer are surgery, chemotherapy and radiotherapy. Surgical annihilation is highly efficient in primary tumours, but it is limited to surgically sizeable and approachable tumours and thus cancer cells may not be wholely evacuated. Chemotherapy is the use of chemical drugs to fight cancer. The systemically administrated drugs circulate in the body to kill cells that divide rapidly, especially cancer cells. It commonly has significant side effects due to drug toxicity to normal cells and is subject to the development of resistance by the cancer cells. Radiation utilizes high energy ionization particles like X-rays, gamma rays or electrons, to damage cells at molecular level and is often used as an integral approach, to exterminate remaining cancer cells after surgery. But, it can cause destruction to the lively/healthy tissues neighbouring the cancer cells or in the lane of radiation beam.
Boron Neutron Capture Therapy (BNCT) is a technique that selectively aims to treat tumour cells sparring the normal cells using Boron compound. Gordon Locher was the first one to propose the principle of BNCT in 1936 and hypothesized that if boron could be selectively concentrated in a tumour mass and the volume then exposed to thermal neutrons, a higher radiation dose to the tumour relative to adjacent normal tissue would be produced [1].
BNCT depends on the following nuclear reaction [2]:
Non-radioactive isotope 10B atoms absorb low-energy (<0.5 eV) thermal neutrons and subsequently breaks up into an α particle (Helium-4) and a recoiled lithium nucleus (7 Li) [Table/Fig-1]. Resultant is the high Linear Energy Transfer (LET) alpha particle, ≈150 keV/μm, 7Li ion, ≈175 keV/μm [3].
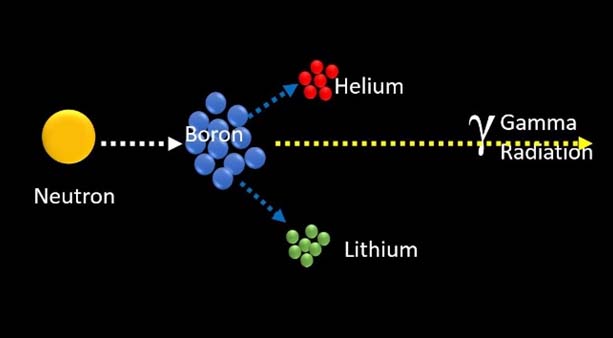
10B + n→7Li + 4He
These particles provide high energy along their very brief pathway (<10μm), Hence, their energy deposition is limited to the diameter of a single cell. Thus, only neoplastic cells with 10B are ravaged following thermal neutron irradiation. Hypothetically, any normal cells abutting the cancer cells are saved from high LET irradiation by 4 He and 7 Li particles [Table/Fig-2].
How BNCT eliminates tumour cells.
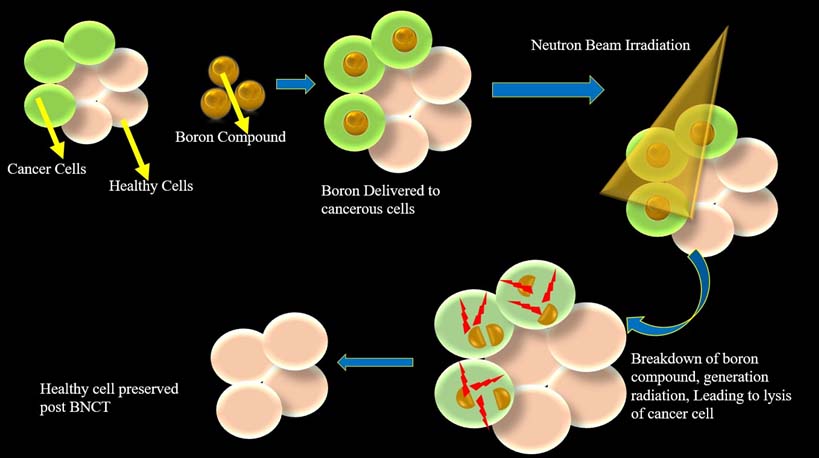
Targeting is primarily achieved by precisely establishing the boron drugs in the tumour rather than by aiming the beam, which provides the explanation for the clinical application of the concept of BNCT.
BNCT integrates the fundamental focusing perception of chemo-therapy and the gross anatomical localization proposition of traditional radiotherapy. The unique outstanding feature of BNCT, is its ability to deposit an immense dose gradient between the tumour cells and normal cells [4]. This serves as the rationale for its clinical implementation in treating malignant cells, thus sparing normal healthy cells.
Historical Milestones in BNCT
The credit of discovery of neutron is attributed to Chadwick in 1932 [5]. Locher in 1936 proposed the principal behind the neutron capture reaction, thus the foundation for BNCT evolved from then [1]. The very first attempt of BNCT was performed in a patient diagnosed with Malignant Glioma in 1951, using the nuclear research reactor presently available in Brookhaven Graphite Research Reactor [6].
Followed by 3 series of treatment process using BNCT was carried out in 40 patients using simple boron compounds, but they were reported with serious side effects like radio-dermatoses of scalp and deep ulcerations [7]. Saltkin mentioned that the outcome of BNCT was similar to traditional radiotherapy, causing cerebral oedema and intractable shock in patients [8]. Sweet WH et al., in 1963 from the reactors of Massachusetts Institute of Technology treated 18 patients using Disodium decahydrodecarborate, which was considered to be less toxic, but was capable of delivering more boron compounds to the cell [9]. Asbury AK et al., noted severe brain necrosis in patients undergoing BNCT [10]. Keeping in mind the potential side effect and toxicity caused by BNCT and the potential harm of using nuclear reactors, USA halted the progress of BNCT in 1961.
Hiroshi Hatanaka in 1968 re-instigated clinical application of BNCT in Japan using Borocaptate Sodium (BSH) by directly exposing the beam to surgically exposed intracranial tumour bed and reported with impressive results of achieving 58% of 5 year survival rate [11–13]. Hatanaka and co-workers reconsidered and revamped the clinical application of BNCT in USA and Europe [3]. In 1987 Mishima from Japan applied BNCT for malignant melanoma using Boronophenylalanine (BPA) as boron compound, this chiselled the clinical implementation of BNCT in treating tumours outside the central nervous system [14]. Slowly and steadily the rebirth of BNCT took place, but was predominantly limited to countries having research reactor facilities capable of delivering epithermal neutron beam, which had better tissue penetration.
BNCT evolved through 60 years of research and clinical progress, but faced n-number of problems including lack of controlled, prospective trials, need of nuclear research reactors for clinical irradiation and disappointment regarding the evolution of ideal boron compounds. The re-emergence of BNCT took pace in the 1990s in USA at Brookhaven [15] and Cambridge, MA [16], then in Europe at Petten [17], in Finland [18], Sweden[19], the Czech Republic [20] and Japan [21,22], and finally in Argentina [23] and Taiwan [24].
Procedure of BNCT:
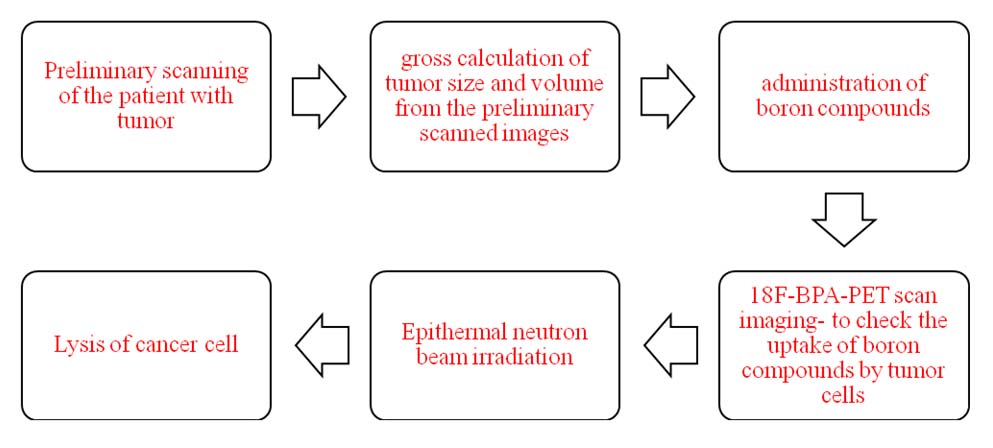
The procedure is illustrated in [Table/Fig-3].
Block diagram representing the procedure for BNCT.
Ideal Properties of Boron compounds: Low systemic toxicity, normal tissue uptake with high tumour uptake, high tumour/brain and tumour/blood concentration ratios (>3-4:1), tumour concentrations of 20-35mg10B/g tumour [25], rapid clearance from blood and normal tissues and persistence in tumour during BNCT.
There are three generation boron compounds presently and there has been a constant refinement in each generation of these boron delivery agents, so that these boron compounds achieve more selective targeting of the tumour cell and provide reasonably low toxicity in the living system.
a) 1st Generation Boron Compounds: Boric acid and some of its derivatives were used as delivery agents in the clinical trials in 1950s and early 1960s. They were elementary chemical compounds, non-discriminatory, had meagre tumour retention, and obtained low tumour/brain ratios.
b) 2nd Generation Boron Compounds: BPA and BSH were two other boron compounds that had emerged out in 1960s. These had significantly lesser toxicity, persisted longer in animal tumours compared with related molecules, and the tumour/brain as well as tumour/blood boron ratios were >1. These compounds are currently being used in many researches and clinical trials.
c) 3rd Generation Boron Compounds: These are stable boron group or cluster connected via a hydrolytically stable linkage with a tumour targeting component or moiety (Boron Carriers). Low and high molecular weight biomolecules such as mitochondria, lysosomes, endoplasmic reticulum, golgi apparatus, nucleoside, sugars (BPA-fructose), porphyrins, liposome and monoclonal antibodies (mAb) have been used as the tumour targeting moiety. These 3rd generation boron compounds tend to work more specifically towards the targeted tumour cell i.e., the tumour cell nucleus and DNA are alluring targets to these agents, also the amount of boron required to produce a lethal effect may be considerably reduced if it is localized within or near the nucleus. These agents are still under research process. BPA-fructose complex was used to treat patients with BNCT in 1994 for treating glioblastoma [26].
Upgradation in Boron Enhancements: There must be sufficient number of boron molecules embedded or incorporated into the tumour cells for their lysis. Thus, to enhance the uptake of boron compounds changes were brought about in the infusion route and rate, for instances in the infusion method intravenous, intra-arterial or direct infusion into the internal carotid artery [27] were tried. Combination of boron with other drugs like mannitol was done [28]. The most promising route that has evolved during the test of time is using tumour targeting moiety and nanoscale drug delivery using liposome and nanoparticles [3].
Neutron Source for BNCT: Neutrons for BNCT must not only be delivered with a high flow rate, but also should have the right amount of energy. The radiation beam which is directed into the tumour bed should have minimal contaminants. The neutron source for epithermal radiation is generated from Nuclear Reactors and Accelerator-Based Neutron Sources (ABNS). Most of these nuclear reactors have already been shut down, or at the verge of closure or have ceased their activities for BNCT trials, only a few are open. Thus, the ultimate hope of BNCT relies on ABNS, which was proposed two decades ago, for BNCT application. ABNS ranging from low-energy electrostatic machines to higher energy cyclotrons and much higher energy Linacs or synchrotrons have been used [3]. The neutron beam produced by ABNS has a low intensity flux compared to nuclear reactor sources, but there exists a possibility of delivering the neutron sources with the desired intensities by many accelerators. Moreover, ABNS is compact and less expensive compared to nuclear reactors. Radiotherapy departments in hospitals are quiet well experienced with accelerators for many years.
Development in Imaging: An important factor that is often marked as a drawback in BNCT is the heterogeneous boron distribution within the tumour, which causes ambiguity in the calculated dose distributions.
Positron Emission Tomography (PET) [29–31] is capable of quantifying boron uptake. In recent years to follow the pharmacological and chemical behaviour of the boron carrier using PET has become an additional stimulus for BNCT. Already 18F-BPA-PET scan images have been routinely used clinically to assess the distribution of boron molecules in patients by many investigators. In fact PET with 18F-labelled BPA is used for patient selection in Japan [32]. In Europe and Finland the use of 18F-BPA and PET was part of the inclusion criteria for BNCT patients [33].
Discussion
The incidence and mortality rates of head and neck cancer are increasing day by day. Aggressive and combined local treatment including surgery and chemo-radiation has been applied to advanced head and neck cancer, but the prognosis for patients with recurrent disease is generally poor. BNCT is emerging as a hopeful tool in treating cancer, by selectively concentrating Boron compounds in tumour cells and then subjecting the tumour cells to epithermal neutron beam radiation which selectively destroys the tumour cells. The unique property of BNCT is that it can deposit a large dose gradient between the tumour cells and normal cells. During the earlier days failure of treatment with BNCT were primarily attributable to; (a) inadequate tumour specificity of boron used as capture agents; (b) insufficient tissue penetrating properties of the thermal neutron beams and (c) high blood boron concentrations that resulted in excessive damage to normal brain vasculature and to the scalp [34,35].
Clinical Trials and Investigations
1. Head and Neck Cancers:1.1 Kankaanranta L et al., reported a cohort of 22 patients with recurrent glioblastoma multiforme, who had recurrence following standard treatment were subjected to BNCT using BPA. It was concluded that BNCT can be used as a rescue therapy in patients with recurrent tumours [36].
1.2 A total of 26 patients (19 squamous cell carcinomas, 4 salivary gland carcinomas and 3 sarcomas) were treated with BNCT following recurrence after standard therapy. Their response rate was 85%, with mean survival time of 33.6 months. The survival periods after BNCT ranged from 1 to 84 months, with Overall Survival (OS) rate of 37% at 2-years and 31.7% at 6-years. It was concluded that BNCT had improved the survival period and quality of life in patients with recurrent HNM [37].
1.3 Kawabata S et al., treated 21 patients with BNCT, using a combination of BPA and BSH. Ten patients were given BNCT only, whereas, rest of the 11 patients were additionally subjected to 20–30Gy fractionated external beam X-Ray irradiation Therapy (XRT) post BNCT. The mean overall survival of the patients was 20.7 months with 2 years survival of 25%. It was concluded that BNCT showed constant endurance of benefit in all cases [38].
1.4 Nineteen patients with recurrent malignant meningioma were subjected to BNCT. Within the observation period there was more than 50% reduction in the tumour mass in 18 out of 19 cases. Presently six patients are still alive. The median survival time post BNCT was reported to be 14.1 months. It is clearly noticeable that BNCT provides a better ground to treat recurrent malignant meningioma cases successfully [39,40].
1.5 Kankaanranta L et al., included 30 patients with inoperable cum locally recurrent cancers of head and neck, who had prior surgery and radiotherapy with or without chemotherapy were subjected to BNCT. Twenty nine patients were evaluated following BNCT, their median Progressive Free Survival (PFS) was estimated to be 7.5 months. Out of 29 cases evaluated, 22 (76%) cases responded well, 6(21%) cases had stable tumour growth from 5.1 and 20.3 months and only one case showed progression. The 2 year OS was 30% and PFS was 20%. There was no evidence of recurrent disease in 27% of cases at the end of 2 years. The most commonly reported grade 3 toxicities were mucositis, oral pain, fatigue followed by bone necrosis (in 3 patients) and soft tissue necrosis (one patient). It can be concluded that BNCT can be used as a treatment modality for locally recurrent and previously irradiated HNC cases [41].
1.6 Ten patients were enrolled for a phase I/II clinical trial of BNCT in Taiwan, for recurrent-late staged head and neck cancer. BNCT was performed with BPA-fructose intravenously in two phases. Their recent findings demonstrated that after a median follow-up of 11.3 months, three patients had shown complete response, three had shown partial response, two showed stable diseases and the rest of the two patients had shown progression of their disease status. Their results clearly expose the effectiveness of BNCT [24,3].
1.7 BNCT was performed in a cohort of 62 patients with a median follow-up of 18.7 months, using either a combination of BSH and BPA or BPA alone. An overall response rate of 58% within 6 months after BNCT procedure was obtained. The median survival time from the time of BNCT reported was 10.1 months; and 1 and 2 years OS rates of 43.1% and 24.2%, respectively. They also stated that BNCT-related morbidity and mortality were acceptably low [4].
1.8 Kato I et al., with the help of epithermal neutron radiation source from Kyoto University Research Reactor (KUR), treated 6 patients with a recurrent Head and Neck Malignancies (HNM) using BNCT, with the combination of BPA and BSH. Their observation were recorded as follows: (1) 10B concentration of Tumour/Normal Tissue ratios (T/N ratio) of PET studies were Squamous Cell Carcinoma (SCC):1.8–4.4, sarcoma: 3.1–4.0, parotid tumour: 3.5; (2) Relative volume (%) of each tumour to the prior was 6–46%; (3) Exceptional reduction (46–100%) of huge tumour, improvement of quality of life and very minimal side effects were recognized in all cases. These results indicate that BNCT represents an upcoming and encouraging treatment modality even for a huge or far advanced HNM [42].
1.9 Haginomori S et al., described the first case of a 42-year-old patient with extensive SCC in the temporal bone recurring after surgery, conventional radiotherapy, and chemotherapy. The patient was treated using planned fractionated BNCT twice with 1-month interval to ensure neutron capture in the deep lesion. They had employed epithermal neutron beam as the neutron source and BPA as the boron compound. The radiological studies performed 6 months after the first BNCT showed remarkable shrinkage in tumour size with no evidence of residual tumour [43].
2. Other Cancers2.1 Yanagie H et al., produced BSH containing water-in-oil-in-water (WOW) emulsion as boron carrier agent in treating recurrent hepatic cancer, as the patient was previously subjected to hepatic arterial chemotherapy. The tumours treated with BNCT showed re-growth in 3 months post BNCT, whereby the patient continued to receive chemotherapy. It was concluded that BSH containing WOW emulsion can be used as an innovative intra-arterial boron carrier for BNCT procedure in recurrent hepatic cancer. He also reported tumour growth suppression 2 months after BNCT procedure using BPA as boron delivery agent in a 72-year-old man for recurrent gastric cancer with left cervical node metastasis [44].
2.2 Suzuki M et al., treated a patient with locally recurrent lung cancer of the chest wall with BNCT. The tumour regressed 7 months after BNCT, with no acute or late adverse effects. The results certainly speaks in favour of BNCT [45].
2.3 The world’s first ever reported case of Extra Mammary Pagets Disease (EMPD) to receive BNCT treatment is credited to Sasaoka S. He treated two patients diagnosed with EMPD, both were above 70 years of age (73 and 75 years old, respectively). Complete regression of the tumour was accomplished in both the cases. What was more noteworthy is that, 12 months after BNCT procedure, neither recurrence nor metastasis has been observed in both the cases [46].
Conclusion
The concept of BNCT though had evolved in 1936, there has been a steady improvement in knowing and understanding the science behind it, working out with the clinical trials and putting it to clinical use. Though may be for now BNCT may not have gained its popularity, but definitely in near future BNCT will be a milestone in the field of radiotherapy for treating cancers.
[1]. Locher GL, Biological effects and therapeutic possibilities of neutronsAm J Roentgenol Radium Ther 1936 36(1):1-13. [Google Scholar]
[2]. International Atomic Energy Agency. IAEA in Austria.2001. Available from: http://www-pub.iaea.org/MTCD/publications/PDF/te_1223_prn.pdf [Last Date of access-11/11/2016] [Google Scholar]
[3]. Moss RL, Critical review, with an optimistic outlook, on Boron Neutron Capture [3] Therapy (BNCT)Appl Radiat Isotopes [Internet] 2014 Available from: http://dx.doi.org/10.1016/j.apradiso.2013.11.109 [Last Date of access-11/11/2016] [Google Scholar]
[4]. Suzuki M, Kato I, Aihara T, Hiratsuka J, Yoshimura K, Niimi M, Boron neutron capture therapy outcomes for advanced or recurrent head and neck cancerJournal of Radiation Research 2014 55(1):146-53. [Google Scholar]
[5]. Chadwick J, The existence of a neutronProc R Soc Lond 1932 A136:692-708. [Google Scholar]
[6]. Farr LE, Sweet WH, Locksley HB, Robertson JS, Neutron capture therapy of gliomas using boron-10Trans Am Neurol Assoc 1954 79:110-13. [Google Scholar]
[7]. Archambeau JO, The effect of increasing exposures of the 10B(n,a)7Li reaction on the skin of manRadiology 1970 94:178-87. [Google Scholar]
[8]. Slatkin DN, A history of boron neutron capture therapy of brain tumoursBrain 1991 114:1609-29. [Google Scholar]
[9]. Sweet WH, Soloway AH, Brownell GL, Boron-slow neutron capture therapy of gliomasActa Radiol Stockholm 1963 1:114-21. [Google Scholar]
[10]. Asbury AK, Ojeman RG, Nielsen SL, Sweet WH, Neuropathological study of fourteen cases of malignant brain tumor treated by boron-10 slow neutron capture radiationJ Neuropathol Exp Neurol 1972 31(2):278-303. [Google Scholar]
[11]. Hatanaka H, Boron-neutron capture therapy for tumors. PrefaceIn: Boron- Neutron capture therapy for tumors 1986 NiigataNishimura Co. Ltd. [Google Scholar]
[12]. Hatanaka H, Clinical results of boron neutron capture therapyBasic Life Sci 1990 54(15):15-21. [Google Scholar]
[13]. Hatanaka H, Sweet WH, Sano K, Ellis F, The present status of boron- neutron capture therapy for tumorsPure Appl Chem 1991 63(3):373-74. [Google Scholar]
[14]. Mishima Y, Ichihashi M, Hatta S, Honda C, Yamamura K, Nakagawa T, First human clinical trial of melanoma neutron capture. Diagnosis and therapyStrahlenther Onkol 1989 165(2–3):251-54. [Google Scholar]
[15]. Chanana AD, Capala J, Chadha M, Coderre JA, Diaz AZ, Elowitz EH, Boron neutron capture therapy for glio- blastoma multiforme: nterim results from the phase I/II dose-escalation studiesNeurosurgery 1999 44(6):1182-93. [Google Scholar]
[16]. Busse PM, Harling OK, Palmer MR, KigerIII W S, Kaplan J, Kaplan I, A critical examination of the results from the Harvard-MITNCT program phase I clinical trial of neutron capture therapy for intracranial diseaseJ Neurooncol 2003 62(1–2):111-21. [Google Scholar]
[17]. Sauerwein W, Zurlo A, The EORTC boron neutron capture therapy group: Achievements and future perspectivesEur J Cancer 2002 38(4):S31-34. [Google Scholar]
[18]. Joensuu H, Kankaanranta L, Seppala T, Auterinen I, Kallio M, Kulvik M, Boron neutron capture therapy of brain tumors: Clinical trials at the Finnish facility using boronophe-nylalanineJ Neurooncol 2003 62(1–2):123-34. [Google Scholar]
[19]. Capala J, Stenstam BH, Sköld K, Rosenschold PM, Giusti V, Persson C, Boron neutron capture therapy for glioblastoma multiforme: Clinical studies in SwedenJ Neurooncol 2003 62(1–2):135-44. [Google Scholar]
[20]. Dbaly V, Tovarys F, Honova H, Petruzelka L, Prokes K, Burian J, Contemporary state of neutron capture therapy in Czech Republic (Part 2)Cesaslov Neurol Neurochir 2002 66-99(1):60-63. [Google Scholar]
[21]. Nakagawa Y, Pooh K, Kobayashi T, Kageji T, Uyama S, Matsumura A, Clinical review of the Japanese experience with boron neutron capture therapy and a proposed strategy using epithermal neutron beamsJ Neurooncol 2003 62(1–2):87-99. [Google Scholar]
[22]. Ono K, Ueda S, Oda Y, Nakagawa Y, Miyatake S, Osawa M, Boron neutron capture therapy for malignant glioma at Kyoto University reactor. In: Larsson B, Crawford J, Weinreich R. (Eds.)Advances in Neutron Capture Therapy 1997 vol.IAmsterdamElsevier Science:39-45. [Google Scholar]
[23]. González SJ, Bonomi MR, Santa Cruz GA, Blaumann HR, Calzetta Larrieu OA, Menéndez P, First BNCT treatment of a skin melanoma in Argentina: Dosimetric analysis and clinical outcomeAppl Radiat Isot 2004 61(5):1101-05. [Google Scholar]
[24]. Liu YWH, Huang TT, Jiang SH, Liu HM, Renovation of epithermal neutron beam for BNCT at THORAppl Radiat Isot 2004 61:1039-43. [Google Scholar]
[25]. Barth RF, Soloway AH, Fairchild RG, Brugger RM, Boron neutron capture therapy for cancer, realities and prospectsCancer 1992 7:2995-3007. [Google Scholar]
[26]. Diaz AZ, Assessment of the results from the phase I/II boron neutron capture therapy trials at the brookhaven national laboratory from a clinician’s point of viewJ Neurooncol 2003 62:101-09. [Google Scholar]
[27]. Sköld K, Stenstam BH, Diaz AZ, Giusti V, Pellettieri L, Hopewell JW, Boron neutron capture therapy for glioblastoma multiforme: Advantage of prolonged infusion of BPA-fActa Neurol Scand 2010 (122):58-62. [Google Scholar]
[28]. Barth RF, Yang W, Rotaru JH, Moeschberger ML, Boesel CP, Soloway AH, Boron neutron capture therapy of brain tumors: Enhanced survival and cure following blood–brain barrier disruption and intracarotid injection of sodium borocaptate and boronophe-nylalanineInt J Radiat Oncol Biol Phys 2003 47:209-18. [Google Scholar]
[29]. Kabalka GW, Smith GT, Dyke JP, Reid WS, Longford CP, Roberts TG, Evaluation of fluorine-18-BPA-fructose for boron neutron capture treatment planningJ Nucl Med 1997 38:1762-67. [Google Scholar]
[30]. Nariai T, Ishiwata K, Analysis and imaging: PET. In: Sauerwein WAG, Wittig A, Moss R, Nakagawa H. (Eds.)Neutron capture therapy: Principles and applications 2012 HeidelbergSpringer-Verlag [Google Scholar]
[31]. Wittig A, Sauerwein WAG, In: Sauerwein WAG, Wittig A, Moss R, Nakagawa H. (Eds.)Neutron capture therapy: Principles and applications 2012 HeidelbergSpringer-Verlag [Google Scholar]
[32]. Yamamoto T, Nakai K, Kageji T, Kumada H, Endo K, Matsuda M, Boron neutron capture therapy for newly diagnosed glioblastomaRadiother Oncol 2009 91(1):80-84. [Google Scholar]
[33]. Kankaanranta L, Seppälä T, Koivunoro H, Saarilahti K, Atula T, Collan J, Boron neutron capture therapy in the treatment of locally recurred head-and-neck cancer: Final analysis of a phase I/II trialInt J Radiat Oncol Biol Phys 2012 (82):e67-75. [Google Scholar]
[34]. Barth RF, Coderre JA, Vicente MG, Blue TE, Boron neutron capture therapy of cancer: Current status and future prospectsClin Cancer Res 2005 11:3987-4002. [Google Scholar]
[35]. Barth RF, Graca M, Vicente H, Harling OK, Kiger WS, Riley KJ, Current status of boron neutron capture therapy of high grade gliomas and recurrent head and neck cancerRadiat 2012 (7):146-67. [Google Scholar]
[36]. Kankaanranta L, Kiovunoro H, Seppälä T, et al. 2008. Outcome of the first twelve patients with locally recurred inoperable head and neck cancer treated in the Finnish head and neck cancer BNCT trial. In: Proceedings of the13th International Congress on Neutron Capture Therapy Florence, Italy, 2012; November2–7, pp. 21–25 [Google Scholar]
[37]. Fuwa N, Suzuki M, Sakurai Y, Nagata K, Kinashi Y, Masunaga S, Treatment results of boron neutron capture therapy using intra-arterial administration of boron compounds for recurrent head and neck cancerBr J Radiol 2008 (81):749-752. [Google Scholar]
[38]. Kawabata S, Miyatake S, Nonoguchi N, Hiramatsu R, Iida K, Miyata S, Survival benefit from boron neutron capture therapy for the newly diagnosed glioblastoma patientsAppl Radiat Isot 2009 67(7–8):S15-18. [Google Scholar]
[39]. Kawabata S. Boron neutron capture therapy for the patients with malignant meningioma. In presented at the15th international congresson neutron capture therapy, Tsukuba, Japan. September 10–14 [Google Scholar]
[40]. Kawabata S, Miyatake S, Boron neutron capture therapy for malignant meningiomas. In: Sauerwein WAG, Wittig A, Moss R, Nakagawa H.(Eds.)Neutron capture therapy: Principles and applications 2012 HeidelbergSpringer-Verlag [Google Scholar]
[41]. Kankaanranta L, Saarilahti K, Makitie A, Valimaki P, Tenhunen M, Joensuu H, Boron neutron capture therapy (BNCT) followed by intensity modulated chemoradiotherapy as primary treatment of large head and neck cancer with intracranial involvementRadiother Oncol 2011 99:98-99. [Google Scholar]
[42]. Kato I, Ono K, Sakurai Y, Ohmae M, Maruhashi A, Imahori Y, Effectiveness of BNCT for recurrent head and neck malignanciesApplied Radiation and Isotopes 2004 61:1069-73. [Google Scholar]
[43]. Haginomori S, Miyatake S, Inui T, Araki M, Kawabata S, Takamaki A, Planned fractionated boron neutron capture therapy for a patient with recurrent squamous cell carcinoma in the temporal bone: A case reportHead Neck 2009 31:412-18. [Google Scholar]
[44]. Yanagie H, Higashi S, Seguchi K, Ikushima I, Oyama K, Nonaka Y, et al. Pilot clinical study of boron neutron capture therapy for recurrent hepatic cancer and gastric cancer. In: Presented at the 15th international congress on neutron capture therapy, Tsukuba, Japan, 2012. September 10–14 [Google Scholar]
[45]. Suzuki M et al. Reirradiation for locally recurrent lung cancer in the chest wall with boron neutron capture therapy (BNCT): A case report. In: Presented at the15th international congress on neutron capture therapy. Tsukuba, Japan, 2012. September. 10–14 [Google Scholar]
[46]. Sasaoka S. The first clinical trial of boron neutron captures therapy using 10B-para-boronophenylalanine for treating extra-mammary Paget’s disease. In: Presented at the 15th International Congress on Neutron Capture Therapy, Tsukuba, Japan, 2012.September, 10–14 [Google Scholar]