Emergence of Hospital Acquired Carbapenem Resistant Non-Fermenters in Teaching Institute
Hariom Sharan1, Neeraj Katare2, Aparna Pandey3, Ganesh Shivmurti Bhatambare4, Trupti Bajpai5
1 Associate Professor, Department of Microbiology, Sri Aurobindo Medical College and Post Graduate Institute, Indore, Madhya Pradesh, India.
2 Student, Department of Microbiology, Sri Aurobindo Medical College and Post Graduate Institute, Indore, Madhya Pradesh, India.
3 Student, Department of Microbiology, Sri Aurobindo Medical College and Post Graduate Institute, Indore, Madhya Pradesh, India.
4 Professor, Department of Microbiology, Sri Aurobindo Medical College and Post Graduate Institute, Indore, Madhya Pradesh, India.
5 Assistant Professor, Department of Microbiology, Sri Aurobindo Medical College and Post Graduate Institute, Indore, Madhya Pradesh, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Hariom Sharan, B-303 Akanksha Apartment, Sri Aurobindo Medical College and Post Graduate Institute, Indore-453555, Madhya Pradesh, India.
E-mail: homsharan@gmail.com
Introduction
Non-Fermenting Gram Negative Bacilli (NFGNB) are emerging now-a-days because of their tendency to colonize various surfaces and inherent resistance to commonly used disinfectants. They are responsible for multi-drug resistant hospital acquired infections. Detection of carbapenem resistance mechanisms is essential for treatment and infection control purpose as can spread to other organisms causing hospital outbreaks.
Aim
To characterize non-fermenters from various clinical samples and to detect different carbapenem resistance mechanisms in meropenem resistant isolates.
Materials and Methods
The prospective study was conducted at Sri Aurobindo Medical College and Post Graduate Institute, Indore over a period of one and half year from December 2014 to May 2016. A total of 1310 samples were collected from Ventilator Associated Pneumonia (VAP), Surgical Site Infection (SSI), Urinary Tract Infection (UTI), septicaemia, Lower Respiratory Tract Infection (LRTI) and middle ear infected patients. Non-fermenters were identified by standard microbiological tests. Meropenem resistance was determined by Kirby-Bauer disk diffusion method and resistant isolates were further tested by Modified Hodge test, Combined disc test and AmpC disc test.
Results
Isolation rate of non-fermenters was 13.82% (181/1310). Colistin, amikacin and imipenem were the antibiotics with maximum sensitivity. Overall meropenem resistance was found to be 44.2% (80/181). Metallo-β-lactamase and AmpC-β-lactamase were produced by 56.82% (25/44) and 72.22% (26/36) of meropenem resistant Pseudomonas and Acinetobacter species respectively.
Conclusion
Detection of carbapenem resistance mechanisms and implementation of antibiotic policy are needed to prevent the emergence of non-fermenter infections.
Combined disc test, AmpC disc test, Modified Hodge test
Introduction
Non-Fermenting Gram Negative Bacilli (NFGNB) are a group of aerobic, non-sporing bacteria that are either incapable of utilizing carbohydrates as a source of energy or degrade them oxidatively [1]. They are found as saprophytes in hospital environment and are responsible for hospital acquired infections like Ventilator Associated Pneumonia (VAP), Septicaemia, Urinary Tract Infection (UTI), meningitis, Surgical Site Infection (SSI) and osteomyelitis particularly in critically ill and immunocompromised patients [2]. Non- fermenters are difficult to treat as they exhibit many mechanisms of drug resistance, non standardization of anti-microbial susceptibility testing for many organisms and poor correlation between in vitro disc diffusion testing results and in-vivo effectiveness of the drugs [3].
There are various mechanisms of carbapenem resistance like mutation in outer membrane proteins, development of efflux pumps and lack of drug penetration [4,5]. Carbapenem resistance can spread to other species, therefore, its monitoring is essential to prevent hospital acquired infections. Combination of antibiotics has shown better response when treating non-fermenter infections [6].
Hence, the present study was to determine the antimicrobial susceptibility pattern and detection of contributing mechanisms in carbapenem resistance development.
Materials and Methods
The present prospective study was carried out over a period of one and half year from December 2014 to May 2016. It was approved by the Institutional Ethical Committee. A total of 1310 samples like endotracheal aspirate, pus, urine, blood, sputum, catheter tip and ear swabs were collected from patients admitted in Intensive Care Unit, medicine, surgery, obstetrics gynaecology, otorhinolaryngology, paediatric wards and burn unit. A total of 925 males and 385 females between 5-75 years of age group, who developed VAP, SSI, UTI, septicaemia, Lower Respiratory Tract Infection (LRTI) and middle ear infection after 48 hours of admission were included in the study. Those who developed these infections before 48 hours of admission were excluded from study.
Direct gram stain was done from all specimens except blood. The specimens were inoculated onto Mac-Conkey’s agar, blood agar and cystine lactose electrolyte deficient (in case of urine sample) medium and incubated at 37°C for 24 hours in 7-10% CO2 concentration. Those organisms which showed non-lactose fermenting colonies on Mac-Conkey’s agar and not acidify the butts of Triple Sugar Iron (TSI) agar were presumptively identified as non-fermenters and confirmed by standard microbiological techniques [1]. All the isolates were tested for anti-microbial susceptibility (Hi-Media Mumbai) by Kirby-Bauer disk diffusion method on Mueller- Hinton agar [7]. Meropenem resistant isolates were proceed to Modified-Hodge test, Combined disc test and AmpC disc test for detection of Carbapenemase, Metallo-β-lactamase and AmpC-β-lactamase.
Quality control strains were Escherichia coli ATCC 25922, for Modified Hodge test- Positive control- Klebsiella pneumoniae ATCC BAA-1705 and Negative control- Klebsiella pneumoniae ATCC BAA-1706.
Modified-Hodge test
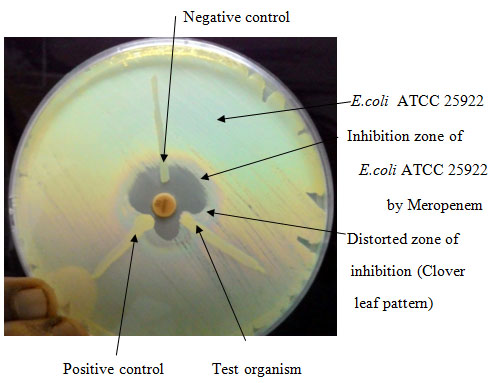
Lawn culture of E.coli ATCC 25922 was made from an overnight culture suspension adjusted to 0.5 McFarland standards on Mueller-Hinton agar. After drying the plate, a 10 μg meropenem disc was placed at the centre and the test strain was streaked from edge of the disc to periphery of plate. The plate was incubated at 37°C for overnight. The presence of distorted zone of inhibition (clover leaf pattern) was interpreted as positive [Table/Fig-1] result [8].
Combined disc test
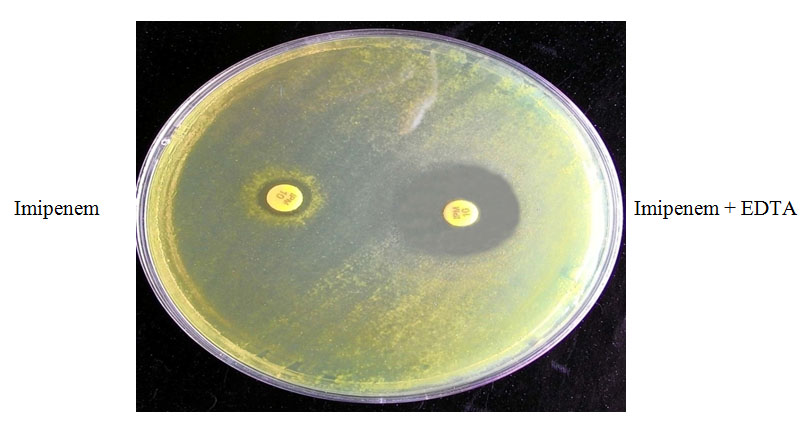
Test organism was inoculated on Mueller-Hinton agar and two 10μg imipenem discs were placed. 10μl solutions (750μg) of Ethylene Diamine Tetra Acetic Acid (EDTA) were added to one of them & incubated the plate at 35°C for 16-18 hours. Metallo-β-lactamase positive [Table/Fig-2] result considered, if zone of inhibition of imipenem + EDTA disc was >7mm than that of imipenem disc alone [9].
AmpC Disc test
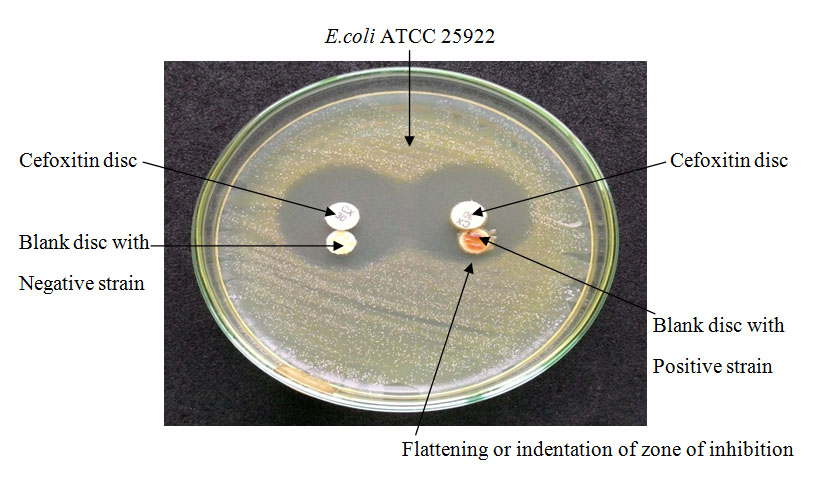
Lawn culture of E.coli ATCC 25922 was made from an overnight culture suspension adjusted to 0.5Mc Farland standards on Mueller-Hinton agar plate. A 30μg cefoxitin disc placed in centre and a blank disc (6mm diameter) which was moistened with sterile normal saline and inoculated with few colonies of test organism, was placed beside the cefoxitin disc almost touching it. The plate was incubated at 37°C for overnight. A flattening or indentation of zone of inhibition of cefoxitin in the vicinity of the disc containing test organism was interpreted as positive [Table/Fig-3] result [10].
Results
Among 1310 patients, 181 showed NFGNB growth, therefore, isolation rate was 13.82%. Majority of non-fermenters were isolated from endotracheal aspirate, but isolation rate was maximum in surgical ward. Predominant isolates were Pseudomonas aeruginosa and Acinetobacter baumannii [Table/Fig-4]. All the isolates showed low sensitivity to piperacillin, ticarcillin and ceftazidime, however, most effective antibiotic in the present study was colistin followed by amikacin and imipenem [Table/Fig-5]. Meropenem resistance was 44.2% (80/181). Metallo-β-lactamase and AmpC β lactamase were produced by 56.82% (25/44) and 72.22% (26/36) of meropenem resistant Pseudomonas and Acinetobacter species respectively [Table/Fig-6].
Non-fermenters isolated from different clinical specimens.
| Specimens | Sample | Pseudomonas aeruginosa | Pseudomonas fluorescens | Pseudomonas stutzeri | Stenotrophomonasmaltophila | Sphingobacteriumspecies | Acinetobacter baumannii | Acinetobacter lwoffii | Total |
|---|
| Endotracheal aspirate | 659 | 45 | 3 | 2 | 3 | 0 | 28 | 4 | 85 |
| Pus | 331 | 25 | 2 | 1 | 2 | 1 | 18 | 2 | 51 |
| Urine | 160 | 12 | 1 | 1 | 1 | 1 | 6 | 1 | 23 |
| Blood | 75 | 4 | 0 | 0 | 0 | 0 | 1 | 0 | 5 |
| Sputum | 60 | 5 | 0 | 0 | 1 | 0 | 2 | 2 | 10 |
| Cather tip | 15 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 3 |
| Ear swab | 10 | 2 | 0 | 0 | 0 | 0 | 1 | 1 | 4 |
| Total | 1310 | 95 | 6 | 4 | 7 | 2 | 57 | 10 | 181 |
Antibiotic sensitivity pattern of Nonfermenters.
| Antibiotics | Conc(μg) | Pseudomonas aeruginosaN (%) | Pseudomonas fluorescensN (%) | PseudomonasstutzeriN (%) | Stenotrophomonas maltophilaN (%) | Sphingobacterium speciesN (%) | Acinetobacter baumanniiN (%) | Acinetobacter lwoffiiN (%) |
|---|
| Amikacin | 30 | 76(80) | 5(83.3) | 4(100) | NT | 2(100) | 35(61.4) | 5(50) |
| Aztreonam | 30 | 30(31.6) | 1(16.7) | 0 | NT | 0 | NT | NT |
| Cefepime | 30 | 57(60) | 2(33.3) | 2(50) | NT | 0 | 15(26.3) | 3(30) |
| Ceftazidime | 30 | 38(40) | 2(33.3) | 2(50) | NT | 0 | 12(21) | 4(40) |
| Ciprofloxacin | 5 | 48(50.5) | 2(33.3) | 3(75) | NT | 0 | 13(22.8) | 4(40) |
| Colistin | 10 | 95(100) | 6(100) | 4(100) | NT | 2(100) | NT | NT |
| Cotrimoxazole | 23.75/1.25 | NT | 1(16.7) | 0 | 7(100) | 1(50) | 16(28) | 3(30) |
| Gentamicin | 10 | 47(49.5) | 3(50) | 3(75) | NT | 1(50) | 23(40.4) | 5(50) |
| Imipenem | 10 | 59(62.1) | 4(66.7) | 3(75) | NT | 2(100) | 40(70.2) | 8(80) |
| Meropenem | 10 | 57(60) | 2(33.3) | 2(50) | NT | 0 | 26(45.6) | 5(50) |
| Piperacillin | 100 | 33(34.7) | 1(16.7) | 2(50) | NT | 0 | 5(8.8) | 2(20) |
| Piperacillin –Tazobactam | 100 / 10 | 58(61) | 2(33.3) | 2(50) | NT | 1(50) | 16(28) | 5(50) |
| Ticarcillin | 75 | 38(40) | 2(33.3) | 2(50) | NT | 0 | 9(15.8) | 3(30) |
| Ticarcillin-Clavulanic acid | 75/10 | 53(55.8) | 4(66.7) | 1(25) | NT | 0 | 18(31.6) | 4(40) |
| Tobramycin | 10 | 49(51.6) | 2(33.3) | 3(75) | NT | 0 | 26(45.6) | 4(40) |
NT- Not Tested
Phenotypic tests in meropenem resistant isolates.
| Bacteria | No. ofIsolates | No. of Meropenem ResistantIsolates | N. of Positive Test |
|---|
| MHT | CDT | AmpC disc test |
|---|
| Pseudomonas aeruginosa | 95 | 38 | 12 | 23 | 18 |
| Pseudomonas fluorescens | 6 | 4 | 1 | 1 | 2 |
| Pseudomonas stutzeri | 4 | 2 | 0 | 1 | 1 |
| Stenotrophomonas maltophila | 7 | 0 | 0 | 0 | 0 |
| Sphingobacterium species | 2 | 0 | 0 | 0 | 0 |
| Acinetobacter baumannii | 57 | 31 | 3 | 5 | 23 |
| Acinetobacter lwoffii | 10 | 5 | 0 | 1 | 3 |
| Total | 181 | 80 | 16 | 31 | 47 |
MHT= Modified Hodge test, CDT= Combined Disc test
Discussion
Isolation rate of non-fermenters was 13.82%, higher than the reports by Benachinmardi et al., and Bruno et al., indicating the rising trend of non-fermenter infections which may be due to survival of organisms in health care setup, disruption of normal flora by excessive use of antibiotics, therapeutic intervention, increased duration of hospital stay, use of steroids and immunosuppressive therapy [11,12]. Non-fermenters were isolated most commonly from endotracheal aspirate indicating respiratory colonization of Pseudomonas and Acinetobacter species. The commonest isolate was Pseudomonas aeruginosa, which is similar to some studies [13,14] and higher isolation rate of Acinetobacter species corresponding to other study [15].
According to sensitivity pattern, antibiotics such as colistin, amikacin and imipenem are available for treatment of non-fermenters. Sensitivity rate was more in Pseudomonas aeruginosa than other species of Pseudomonas and resistance rate was higher in Acinetobacter baumannii than Acinetobacter lwoffii in most of the antibiotics. In the present study, amikacin sensitivity was 80% for Pseudomonas aeruginosa which is contrast to the finding of Kumar R et al., who have reported the same to be 32% [16]. Imipenem resistance was 62.1% in Pseudomonas aeruginosa whereas Malini et al., detected it 6% only [14]. In our study, high percentage of resistance has been detected against beta-lactam (piperacillin, ticarcillin) and cephalosporin (ceftazidime). This leaves us with limited therapeutic options, thereby, resulting in resistance among other group of antibiotics and such resistance have a potential for rapid spread since, they are usually plasmid mediated. The present study showed higher resistance in ciprofloxacin which can be explained by the fact of non-judicious use and easy availability even without prescription. Meropenem resistance was 40% in Pseudomonas aeruginosa and 54.39% in Acinetobacter baumannii which may be up to 42.7% [17] and 90.3% [18] indicate increasing trend of carbapenem resistance in non-fomenters. A total of 56.82% Metallo beta lactamase and 72.22% AmpC beta lactamase were detected among Pseudomonas and Acinetobacter isolates. Among carbapenem resistance acquisition, AmpC-beta-lactamase mechanism is more common than Metallo beta lactamase and carbapenemase other than Metallo beta lactamase in our health care setup. Overall our study has implicated the severity of carbapenem resistant non-fermenters, which are affecting the infection control activities among hospital setup.
Limitation
Numbers of samples are less. Our study was limited to the detection of carbapenemase production only among carbapenem resistant non-fermenters isolated from inpatients.
Conclusion
Carbapenem resistance rate was more in Acinetobacter than Pseudomonas species. Our study highlights that no single phenotypic test is sufficient for detection and differentiation of carbapenem resistance among non-fermenters. Detection of carbapenem resistance (Metallo-β-lactamase, Carbapenemases other than MBL and AmpC β lactamase) is essential because they limit treatment options and can spread to susceptible bacteria by gene transfer. Antibiotics according to susceptibility pattern and infection control measures are necessary in prevention of emergence of non-fermenters in our teaching institute. However, higher resistance to beta lactam and cephalosporin is one of the alarming sign in our region.
NT- Not Tested
MHT= Modified Hodge test, CDT= Combined Disc test
[1]. Winn W Jr, Allen S, Janda W, Koneman E, Procop G, Schreckenberger P, Nonfermenting gram negative bacilliIn: Koneman’s color atlas and textbook of Diagnostic Microbiology 2006 6th edUSALippincott Williams and Wilkins Company:305-91. [Google Scholar]
[2]. Rajendra D, Ramana BV, Chaudhury A, Spectrum of non fermenting gram negative bacilli infection (excluding Pseudomonads) in a tertiary care hospitalInt J Biol Med Res 2012 3(3):1902-04. [Google Scholar]
[3]. Arora S, Gautam V, Ray P, Changing susceptibility patterns of nonfermenting gram negative bacilliIndian J of Med Microbiol 2012 30(4):485-86. [Google Scholar]
[4]. Rumbo C, Gato E, Lopez M, Ruiz DAC, Fernandez CF, Martinez ML, The contribution of efflux pumps, porins and β lactamases to multi-drug resistance in clinical isolates of Acinetobacter baumanniiAntimicrob Agents Chemother 2013 57(11):5247-57. [Google Scholar]
[5]. Meletis G, Exindari M, Vavatsi N, Sofianou D, Diza E, Mechanisms responsible for the emergence of carbapenem resistance in Pseudomonas aeruginosaHippokratia 2012 16(4):303-07. [Google Scholar]
[6]. Sukumaran J, Sriram L, Sumathi G, Non fermentative gram negative bacilli– characterisation and antibiotic resistant pattern study from a tertiary care hospitalIndian Journal of Basic and Applied Medical Research 2014 3(4):227-32. [Google Scholar]
[7]. CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Twenty -Fifth Informational Supplement. CLSI document M100-S25. Wayne, PA: Clinical and Laboratory Standards Institute; 2015 [Google Scholar]
[8]. Kumar KM, Saikumar C, Detection of metallo β lactamase production in Pseudomonas aeruginosa by various phenotypic methodsResearch Journal of Pharmaceutical, Biological and Chemical Sciences 2016 7(1):1756-59. [Google Scholar]
[9]. Ranjan S, Banashankari GS, Babu PR, Evaluation of phenotypic tests and screening markers for detection of metallo β lactamases in clinical isolates of Pseudomonas aeruginosa: A prospective studyMed J DY Patil Univ 2015 8:599-605. [Google Scholar]
[10]. Willems E, Verhaegen J, Magerman K, Nys S, Cartuyvels R, Towards a phenotypic screening strategy for emerging β lactamsases in gram negative bacilliInt J Antimicrob Agents 2013 41(2):99-109. [Google Scholar]
[11]. Benachinmardi KK, Padmavathy M, Malini J, Naveneeth BV, Prevalence of non-fermenting gram negative bacilli and their in vitro susceptibility pattern at a tertiary care teaching hospitalJ Sci Soc 2014 41:162-66. [Google Scholar]
[12]. Bruno D, Nishino MK, Priore WN, Remus PR, Do Carmo AA, Stefanello VB, Prevalence of non fermenting gram negative bacilli among inpatients from Porto Alegre-RSJ Bras Patol Med Lab 2011 47(5):529-34. [Google Scholar]
[13]. Gokale SK, Metgud SC, Characterization and antibiotic sensitivity pattern of nonfermenting gram negative bacilli from various clinical samples in a tertiary care hospital, BelgaumJ Pharm Biomed Sci 2012 17(14):1-5. [Google Scholar]
[14]. Malini A, Deepa EK, Gokul BN, Prasad SR, Nonfermenting gram negative bacilli infections in a tertiary care hospital in Kolar, KarnatakaJ Lab Physicians 2009 1(2):62-66. [Google Scholar]
[15]. Jaggi N, Sissodia P, Sharma L, Acinetobacter baumannii isolates in a tertiary care hospital: Antimicrobial resistance and clinical significanceJ Microbiol Infect Dis 2012 2(2):57-63. [Google Scholar]
[16]. Kuma R, Srivastva P, Rishi S, Dahiya SS, Hemwani K, Nirwan PS, Detection and antimicrobial susceptibility pattern of Pseudomonas aeruginosa isolates in various clinical samples with special reference to Metallo beta lactamase from a tertiary care hospital in Jaipur, IndiaNational Journal of Medical Research 2014 4(2):128-31. [Google Scholar]
[17]. Manoharan A, Chatterjee S, Mathai D, Detection and characterization of metallo beta lactamases producing Pseudomonas aeruginosaIndian J Med Microbiol 2010 28(3):241-44. [Google Scholar]
[18]. Guckan R, Kilinc C, Demir AD, Capraz A, Yanik K, Antimicrobial susceptibility of Acinetobacter baumannii complex isolated from different clinical samples in a tertiary care hospitalJ Antibiot Res 2015 1(1):1-5. [Google Scholar]