Viruses are one of the deadliest organisms known to cause large epidemics and pandemics in various parts of the world. Diseases caused by viruses have assumed great public health significance in the recent past [1]. Out of the 20 emerging/re-emerging infections all over the world, 14 are of viral origin [2]. In India, during the last 30 years, 30 different outbreaks have been recorded, of which 21 have been due to different viruses [2]. The concepts of public health have experienced a new paradigm as a consequence of these emerging viruses. Besides health, the viruses have also influenced the social and economic fabric of India [2].
Age old viruses causing dengue fever and chikungunya fever have emerged as major public health issues. Both these diseases are vector-borne and have a complex epidemiology [3–5]. Laboratory tools are essential for their diagnosis and for differentiating them from other fevers for institution of rational and specific therapy [6]. Hepatitis A virus (HAV) and Hepatitis E virus (HEV) are responsible for enterically-transmitted acute viral hepatitis. Infection with both HAV and HEV are endemic in developing countries [7]. In addition, there is a high occurrence of risk factors for hepatitis B and C virus infection also in India. The impact of these infections is emerging in India due to flaws in India’s blood-banking system and non-execution of international standards concerning blood transfusion [8,9]. Herpes Simplex Virus (HSV) types 1 and 2 cause genital herpes infections and are the most common cause of genital ulcer disease worldwide. Although most herpes infections are asymptomatic or very mild but they can be transmitted to fetus and show associations with other STDs and cervical cancer [10].
Amritsar, occupying North-western regions of Punjab state, is one of the most primitive and alluring cities in India. It acted as the major corridor for travellers coming from Central Asia to India via overland route and soon became the centre of various commercial activities. A gradual increase in population size has been experienced since the last century. The existing environmental, socioeconomic and demographic factors in the region converged leading to disproportionate burden of infectious diseases in Amritsar [11,12].
The epidemiology of the deadly viral diseases needs to be studied in depth. There is paucity of published data on epidemiology of the viral infections in India, particularly in Punjab. The Viral Research and Diagnostic Laboratory (VRDL) at Government Medical College (GMC), Amritsar conducted the initial diagnosis during the outbreak/epidemic in Amritsar and neighbouring districts including Gurdaspur, Jalandhar, Kapurthala, Pathankot and Tarn Taran. The laboratory deals with all common viruses existing in the region classified according to their routes of transmission: respiratory: measles, rubella, mumps, influenza viruses; intestinal: hepatitis A, E; vector borne: dengue, chikungunya, Japanese encephalitis; body fluids: hepatitis B and C [2]. The present paper reports the various occurrences and outbreaks of viral diseases in Amritsar and neighbouring district of Punjab, along with the demographic and geographical data of the patients. The study reports a total of nine outbreaks comprising of ChikV, DenV, HAV and HEV epidemics in the studied population.
Materials and Methods
The present study was a cross-sectional study. The blood samples from a total of 5781 patients suspected of chikungunya, dengue, hepatitis A, hepatitis B, hepatitis C, hepatitis E, herpes simplex-1 or herpes simplex-2 visiting the Guru Nanak Dev hospital (affiliated to Government Medical College (GMC), Amritsar (Punjab), India) were received at the VRDL located in GMC, Amritsar, for a period from January 2015 till April 2016. Suspected samples have been received from Amritsar, Gurdaspur, Jalandhar, Kapurthala, Pathankot and Tarn Taran districts. In addition few random cases have also been received from other districts such as Ferozepur, Hoshiarpur, Ludhiana, Moga and Patiala which have been clubbed together as ‘others’ group for district-wise comparison.
The present study is in compliance with the ethical standards of committee responsible for human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
All the samples were accompanied with demographic and geographical details of the patient. Patients of all age groups with positive viral infection based on serological assays, Enzyme Linked Immune Sorbent Assay (ELISA) test were included in the present study. The ELISA kits used were as such: CHIKV - Panbio, DENV – Panbio (NS1), TMB (IgM); HAV - Bioneovan, HBV - J. Mitra (HBsAg), HCV - J. Mitra, HEV - Bioneovan, HSV1 – EuroImmum; HSV2 - EuroImmum. Patients with positive viral antibodies but residing outside Punjab were excluded.
About 5 ml of whole blood sera were received in an ice box maintained at 2-8°C and processed within 24-48 hours. The samples were tested for IgM antibodies of Chikungunya Virus (ChikV), Dengue Virus (DenV), Hepatitis A Virus (HAV), Hepatitis B Virus (HBV), Hepatitis E Virus (HEV), Herpes Simplex Virus-1 (HSV-1) and Herpes Simplex Virus-2 (HSV-2) using IgM antibody capture ELISA kits. For patients suspected of DenV infections nonstructural protein 1 (NS-1) antigen (fever <5days) was also performed [13]. Hepatitis C Virus (HCV) testing was done for presence of IgG antibodies. All the tests were carried out following the manufacturer’s instructions.
Statistical Analysis
The data was analysed using Microsoft Office Excel ver. 2007. The percentage and proportions for every variable was evaluated. The data was analysed statistically, using Chi-square tests. A p-value <0.05 was considered statistically significant.
Results
In the present study, a total of 5781 subjects suspected for ChikV, DenV, HAV, HBV, HCV, HEV, HSV1 and HSV2 have been tested and out of these 1790 cases have been reported to be positive for one or more of these viral infections [Table/Fig-1]. It is noteworthy that the DenV is predominant in the studied districts with a total of 1538 subjects presenting the anti-DenV antibodies. It is followed by HCV (120), HEV (70), HBV (28), ChikV (17), HAV (7), HSV1 (3) and HSV2 (2).
Distribution of serologically tested positive cases out of the total suspected subjects.
| Name of Virus for Which Investigation was Done | Total Cases | Positive Cases (%) |
|---|
| ChikV | 20 | 17 (85) |
| DenV | 2709 | 1538 (56.77) |
| HAV | 92 | 7 (7.61) |
| HBV | 1320 | 28 (2.12) |
| HCV | 1305 | 120 (9.20) |
| HEV | 94 | 70 (74.47) |
| HSV1 | 5 | 3 (60) |
| HSV2 | 4 | 2 (50) |
| Total | 5781 | 1790 |
The male group had higher prevalence of almost all the studied viruses; ChikV (52.94%), DenV (67.1%), HAV (71.43%), HBV (67.86%), HCV (82.5%), HSV2 (100%), as compared to their female counterparts, except HEV (48.57%) and HSV1 (0%). However, the gender specific difference was statistically significant (p=0.0001) only for HCV. The mean age laid between 20 years (HAV) and 46.94 years (ChikV) and the median laid between 19.5 years and 46 years (HSV2) [Table/Fig-2].
Baseline demographic characteristics of the surveyed patients.
| Parameter | ChikV | DenV | HAV | HBV | HCV | HEV | HSV1 | HSV2 |
|---|
| N | 17 | 1538 | 7 | 28 | 120 | 70 | 3 | 2 |
| Sex |
| Male (%) | 9 (52.94) | 1032 (67.1) | 5 (71.43) | 19 (67.86) | 99 (82.5) | 34 (48.57) | 0 (0) | 2 (100) |
| Female (%) | 8 (47.06) | 506 (32.9) | 2 (28.57) | 9 (32.14) | 21 (17.5) | 36 (51.43) | 3 (100) | 0 (0) |
| Chi-square | 0.194 | 1.890 | 0.171 | 0.237 | 14.924 | 0.029 | 0.052 | 1.333 |
| p-value | 0.6595 | 0.1692 | 0.6788 | 0.6263 | 0.0001 | 0.8656 | 0.8195 | 0.2482 |
| Age |
| Mean | 46.94 | 39.63 | 20 | 34.93 | 39.13 | 27.04 | 41 | 46 |
| Median | 45 | 40 | 19.5 | 29.5 | 38 | 26 | 35 | 46 |
| SD | 15.07 | 16.69 | 11.62 | 14.92 | 13.22 | 9.15 | 21.63 | 8.49 |
| Range | 28-76 | 14-86 | 6-48 | 19-70 | 20-78 | 12-52 | 23-65 | 40-52 |
It is evident that the youngest age group (0-20 years) did not present with any case of ChikV, HSV1 or HSV2 infection. However, HAV largely affected this group with 6 out of total 7 positive cases. The 21-40 years group appeared to be the most susceptible age group for nearly all the studied viral infections (DenV-632, HBV-19, HCV-66, HEV-55, HSV1-2, HSV2-1). A significant number of cases have also been reported in the oldest age group (>60 years) with ChikV (4), DenV (190), HBV (3), HCV (4) and HSV1 (1) infections [Table/Fig-3].
Age-wise distribution of serologically tested positive cases.
| Virus | 0-20 (years) | 21-40 (years) | 41-60 (years) | >60 (years) |
|---|
| ChikV | 0 | 6 | 7 | 4 |
| DenV | 148 | 632 | 568 | 190 |
| HAV | 6 | 0 | 1 | 0 |
| HBV | 2 | 19 | 4 | 3 |
| HCV | 2 | 66 | 48 | 4 |
| HEV | 10 | 55 | 5 | 0 |
| HSV1 | 0 | 2 | 0 | 1 |
| HSV2 | 0 | 1 | 1 | 0 |
A district-wise distribution of positive cases for tested viruses revealed that maximum cases of ChikV, DenV, HAV, HBV, HCV, HEV and HSV-2 were reported from Amritsar district. However, maximum HSV1 cases were reported from Gurdaspur. A significant number of DenV cases have been reported from Kapurthala and other districts besides Amritsar [Table/Fig-4].
Percentage of positive cases detected from various surveyed districts.
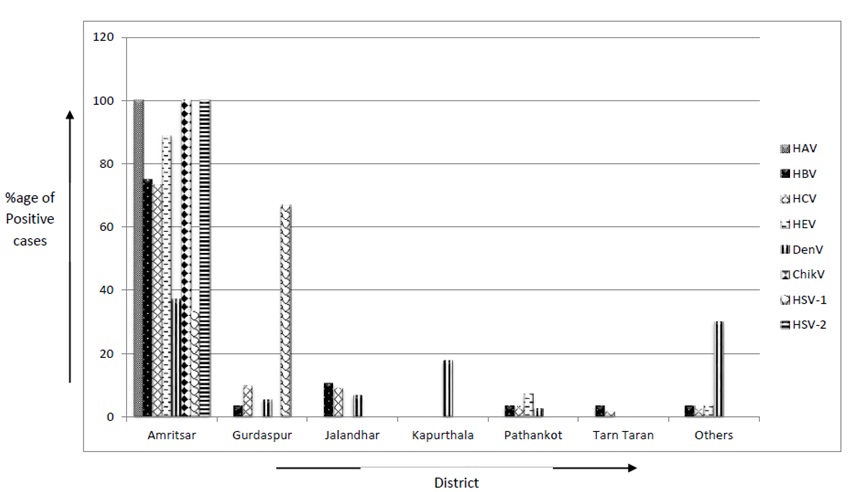
The largest outbreak encompassing 1335 DenV cases was diagnosed from the Amritsar district. The DenV outbreak in Tarn Taran followed with 168 cases, while Gurdaspur trailed with 154 positive cases. The Jalandhar district showed four predominant outbreaks for HAV and HEV. Between the two, HEV prevalence was much higher (22 out of 34 suspected patients) as compared to HAV (1 out of 34 suspected patients). Gurdaspur also reported HAV and HEV outbreaks consisting of 31 HEV positive cases out of 33 suspected patients [Table/Fig-5].
Number of outbreaks investigated.
| District | Outbreak ID | No. of Patients | Virus tested | Positive cases |
|---|
| Amritsar | 1 | 15 | ChikV | 15 |
| Amritsar | 2 | 1335 | DenV | 996 |
| Gurdaspur | 3 | 33 | HAV | 4 |
| HEV | 31 |
| Gurdaspur | 4 | 189 | DenV | 154 |
| Jalandhar | 5 | 12 | HAV | 1 |
| HEV | 5 |
| Jalandhar | 6 | 6 | HAV | 0 |
| HEV | 4 |
| Jalandhar | 7 | 10 | HAV | 0 |
| HEV | 10 |
| Jalandhar | 8 | 6 | HAV | 0 |
| HEV | 3 |
| Tarn Taran | 9 | 192 | DenV | 168 |
Discussion
Chikungunya virus was first isolated in Tanzania in 1953 [6]. Since then a number of periodic outbreaks cropped up in entire Africa and also in Southeast Asia, however, they were typically self-limiting and did not extend to broader geographical areas. It reached India in 2006 where more than 1,000,000 suspected cases were reported [6]. However, in present study only 20 chikungunya suspected cases visited the hospital. Out of these 17 showed positivity for antibodies against ChikV and all of them hailed from Amritsar district. This might be due to difficulty in differentiating between chikungunya and dengue infections [14].
The ChikV was slightly more prevalent in males (52.94%) as compared to the females (47.06%). These findings were much similar to the pattern shown by Kalawat et al., [4] but in contrast with studies by Balasubramaniam SM et al., and Dwibedi B et al., [15,16]. ChikV infection is more common in adults (>20 years) as no case was diagnosed in the youngest age group (0-20 years) and 13 out of total 20 positive cases were in the age group of 20-60 years. Similarly Mohanty I et al., found that the age group 15-45 years was mostly affected whereas lower number of cases were seen from elderly persons >60 years [17].
The last decade experienced the dengue infection in pan-India proportions. Multiple outbreaks and mortality have been recorded from all over India extending from Punjab, Haryana and Uttar Pradesh in the north till Andhra Pradesh, Tamil Nadu and Karnataka in the south; also from Gujarat and Rajasthan in the west till West Bengal in the east [18]. The DenV is the most common mosquito borne viral infection undergoing rapid spread worldwide. In the past 20 years very less data of DenV infections have been documented from the Northern India [3,5], probably due to lack of diagnostic facilities. The present results show that the overall seropositivity of dengue suspected cases is 56.77% (1335 out of 2709). This is relatively higher than other Indian studies; Ukey PM et al., reported 31.3% patients to be serologically positive for dengue infection from Central India [3] and a Tamil Nadu based study reported 32.1% seropositivity [19]. It indicates an increase in dengue virus activity, raising the question whether dengue is emerging/re-emerging as a major health problem in Amritsar.
In this study the male group has higher prevalence of DenV (67.1%) than the female group (32.9%). The majority of previous DEN outbreak studies also documented that males outnumbered the females in DenV positivity [19–22]. However, this may be the representation of all the patients who visited the health care system to seek medical care rather than the truly infected population. The 21-40 years group appears to be the most susceptible age group for DenV in the present study with 632 out of 1538 positive cases from this group. Similar findings were observed in the study done by Sandhya BK et al., and Sood S [19,22]. However our findings are contradictory to some of the studies done elsewhere in India, who reported the paediatric age group as the most vulnerable when compared with adults [23].
Hepatitis A virus is widely spread and has inverse relationship with socioeconomic status, affecting mostly the areas with poor sanitation conditions [7]. Punjab is generally considered an area of low endemicity for hepatitis A in comparison to other Indian states [7]. In the current study the seroprevalence of HAV is 7.61% (7 out of 92 suspected cases). In the present study, the decreasing trend of HAV prevalence is in parallel to shifting patterns of sero-prevalence in South-East Asia and China over the past 20 years reflecting environmental hygiene and improved living standards [7,24].
Sex wise distribution shows that males have higher incidence of HAV (71.43%) than females. This might be because major route of transmission of HAV is feco-oral route with poor sanitation and contaminated food/water supplies and as male go out more for work, so chances of eating outside also increase as shown by other studies [7,25,26]. Previous HAV seroprevalence studies from India reported anti-HAV antibodies in 90% of Indian children in the age group of 5-10 years [27]. The present data shows that anti-HAV antibody prevalence is the highest (96%) in children (0-20 years). The seroprevalence of HEV is quite high as it was observed in 70 out of 94 suspected cases (74.47%). HEV mainly affects young adults of 15-40 years of age and relatively spares children [7]. In India, HEV is responsible for 50-70% of all cases of sporadic acute viral hepatitis [25–27] and also, is responsible for large outbreaks with source of infection mainly being contaminated water supplies [7]. The occurrence of large epidemics of HEV in disease-endemic areas, as it is the case in the present study, suggests the possibility of doubtful protection from the antibody, gradual decline in the protective level of the antibody or infection from divergent strains of the virus [25].
Most disease-endemic areas such as India, report a quite low seroprevalence of HEV in children aged 10 years and below, but rises to 30% among adults >25 years of age [25]. The present study points towards an overall high endemicity of HEV infection (78.78%) in adults (21-40 years) and a significantly lower seroprevalence in the age groups <21 years and >40 years. Similar findings were reported by other researchers [27,27]. This was in sharp contrast to few other studies [28]. These differences might be due to either the differences in the diagnostic tests used or varying environmental conditions in different geographical areas.
The Jalandhar district shows four predominant outbreaks for HAV and HEV. Between the two, HEV prevalence was much higher (22 out of 34 suspected patients) as compared to HAV (1 out of 34 suspected patients). Gurdaspur also reported HAV and HEV outbreaks consisting of 31 HEV positive cases out of 33 suspected patients.
HBV and HCV infections are major issues associated with blood transfusion. The developed countries have taken stern measures for preventing transfusion-transmitted infections by limiting unnecessary transfusions, systematic screening of all donated blood for infections, using routine voluntary donors only and not including donors with specific risk factors. However, no such screening methods are employed in most of the developing countries, therefore, the risk of transfusion-transmitted infection remains high [29].
Hepatitis-B is found throughout the world and it has no seasonal distribution. The reported prevalence of carrier in different population varies widely from as low as 0.1% in the advanced countries to 20% in the developing nations [30]. The current study shows 2.12% seroprevalence of HBV among suspected cases visiting the hospital. The frequency of HBV seropositivity has been found to be lower than that reported in other studies [31,32].
With respect to sex related prevalence, this study shows that the prevalence of HBV was 67.86% in males and 32.14% in females. This finding of the present study is in concern with other study, which demonstrated higher prevalence of HBV among the male population [33]. The present study reveals that HBV prevalence was highest in adult age group, 21-40 years. This is comparable with some other Indian studies [34,35]. Higher prevalence in older age groups might be linked to birth cohort effect or iatrogenic factors such as use of unsterilized kits for vaccinations. Moreover, the hepatitis-B vaccines have been introduced lately, therefore the older age group population having a higher prevalence of HBsAg detected in the present study is presumptively due to lack of immunization against the disease in their times. In the present study, declining seropositive rates in individuals aged 41 years or over was observed for both genders. Self-selection due to persistent HBV infection may partly account for such tendencies [36].
The seroprevalence of HCV in present study was 9.2% of the total suspected cases visiting the hospital. General prevalence of HCV was 0.5% to 1% and there are about 15 million HCV carriers in India [37]. Prevalence of anti-HCV in blood donors in developed countries range from 0.4-2% [8].
The male group has predominance of HCV (82.5%) as compared to the female group (17.5%) and the gender specific difference is statistically significant (p=0.0001). Similar results were reported in several other studies [9,38]. A sex-wise difference in seroprevalence might be due to difference in the risk behaviour. It is also observed that the HCV infection is more prevalent in adults aged between 21 to 60 years.
Infections due to HSV are extremely common. In the current study HSV-1 has been detected in 60% and HSV-2 in 50% of suspected cases. The higher seropositivity of HSV-1 in females and HSV-2 in males in this study might probably be due to limited sample size. HSV seropositivity is highest in the young age group of 21-40 years because of their sexually active life [39]. Adolescents are known to be at increased risk of acquiring STIs because of fewer protective antibodies and increased susceptibility of cervix. Similar results are reported by another study [39].
Overall this study provided a picture of common viruses prevalent in the region. The individuals infected with the viruses are also associated with number of co-morbidities. The heavy burden of infectious disease indicates further investigation into any potential biologic interactions between these common viral infections.
Limitation
Firstly sample size was low for some viruses (ChikV, HAV, HEV, HSV-1, HSV-2), more subjects would yield more reliable results. Secondly the quality of laboratory data may also be imperfect because most laboratory tests fail to correctly identify some percentage of true positives and negatives and lastly confirmation of the presence of the various viruses with a nucleic acid based techniques (such as Polymerase Chain Reaction) would have been useful. Nonetheless, the present results are consistent with other studies, and are relevant for targeting public health interventions.
Conclusion
India is one of the most populous countries, such that even low prevalence accounts to a large number of infected people. Stringent measures should be taken on urgent basis including dissemination of information including public awareness, educational and motivational programs, better donor recruitment, promoting voluntary blood donations, safe sexual practices, proper sterilization of instruments and proper disposal of contaminated material. Finally, a binational approach to surveillance and prevention is needed to address public health problems. Emergence and re-emergence of diseases is a common phenomenon in humans since ages. Considering the present scenario of viral infections in Amritsar and its neighbouring districts of Punjab, enhanced surveillance is recommended so as to accurately monitor the trends of various viral diseases prevalent in the region and to find out the yet undiagnosed viral infections. VRDL established in GMC, Amritsar has become instrumental in detection of emerging and re-emerging viral diseases including outbreak investigation of communicable diseases. Timely action with the help of adequate information and capacity to diagnose the viruses might help is saving numerous lives.