Introduction
Nosocomial infections have been considered as a major health problem causing incremental morbidity, mortality and costs of therapy.
Aim
This retrospective study was initiated with aim to analyse the comparative efficacy of a novel Antibiotic Adjuvant Entity (AAE), a combination of ceftriaxone + sulbactam + disodium edetate and meropenem in combination with colistin, for the management of Multi Drug Resistant (MDR) nosocomial Gram-negative bacterial infections.
Materials and Methods
Case history sheets of patients with documented MDR nosocomial Gram-negative infections who received either AAE or meropenem in combination with colistin for management of infections over a period of 3 years (November 2012 – October 2015) were included in the study. Data related to clinical management, demographics, vital signs and laboratory parameters along with prior antibiotic therapy, dose and clinical outcomes were evaluated thoroughly to analyse the clinical benefits of this new AAE+ colistin therapy for management of MDR nosocomial infections.
Results
Out of 115 patients short listed for the study, 52 patients had received AAE + colistin therapy and 63 patients have received meropenem + colistin. AAE + colistin therapy resulted in significantly higher efficacy (86.53%) as compared to meropenem + colistin (63.49%). A rising trend in clinical cure rates was observed in AAE based combination therapy in contrast to the decreasing trend in meropenem based combination therapy. A progressive decline in clinical cure rates was observed in meropenem treated group over a period of 3 years due to rising carbapenemases and multiple resistance by pathogens, where as AAE maintained the same efficacy.
Conclusion
The AAE + colistin therapy has shown better bacteriological and clinical efficacy as compared to meropenem + colistin in the management of various nosocomial MDR Gram-negative infections. A significant number of meropenem failure patients responded to the AAE therapy highlighting the new hope to spare carbapenems.
Introduction
Nosocomial infections represent a major health problem and various studies have shown that these infections are responsible for the incremental morbidity, mortality and costs of the therapy [1]. Lower Respiratory Tract Infections (LRTIs), along with Blood Stream Infections (BSIs) are amongst the most prevalent nosocomial infections [2–4]. LRTIs are thought to be leading cause of death all over the world [5]. Mortality associated with BSIs may range from 20 to 50% and depends on several factors, including the pathogen and host [6]. Numerous pathogens including Escherichia coli,Pseudomonas aeruginosa, Proteus mirabilis, Klebsiellapneumoniae, Enterobacter spp., and coagulase-negative Staphylococci are responsible for LRTIs and BSIs [7,8]. Among various classes of drugs, β-lactams are one of the most frequently prescribed empirical antimicrobial drugs for the treatment of these infections [9]. However, in recent years, rise in resistance to β-lactam drugs has been noticed because of the Extended Spectrum β-Lactamases (ESBLs) and Metallo-β-Lactamases (MBLs) enzymes which hydrolyse most of the β-lactam antibiotics [10–15].
As a result of the increasing resistance towards antibiotics over the past few years, it is no wonder that we are now facing the prospect of losing the battle against many bacterial diseases. Combination therapies must be developed which could be used empirically in critically ill patients to ensure clinical cure and safety.
A new approach to improve the existing antimicrobial agents is the use of Antibiotic Adjuvant Therapy (AAE). A new AAE (ceftriaxone, sulbactam with adjuvant EDTA) has been reported to have proven efficacy in a wide range of infections [16,17]. This retrospective observational study has been performed to evaluate the best choice of antibiotic combinations to be used in the management of multi-drug resistant nosocomial infections and to evaluate this new combination against meropenem.
Materials and Methods
Present study was a retrospective observational analysis of the data collected from Jawaharlal Nehru Medical College, Aligarh from November 2012 to October 2015. The study was carried out in accordance with the ethical principles of the Declaration of Helsinki and to the current norm for observational studies. Due to the retrospective study design, informed consent was not deemed necessary. Case history sheets of all the patients were reviewed and relevant information like patient’s age, gender, co-morbidities, antibiotic therapy, dose and duration, switch of antibiotic therapy and the reasons for the shift and length of the hospital stay were recorded. Patients meeting one of the following criteria were considered for the study. The criteria for the patient selections were; 1) Patients diagnosed (defined by clinical investigations and relevant signs and symptoms) with either of Community Acquired Pneumonia (CAP), Hospital Acquired Pneumonia (HAP), Ventilator Associated Pneumonia (VAP) or BSIs, due to MDR nosocomial Gram-negative pathogens; 2) Patients who have been hospitalized for more than 5 days; 3) Patients with recent failure history of multiple antibiotic procedures; 4) Patients with identified baseline and/or super-infection culture with resistance to multi-drugs; and 5) Patients with multiple co-morbidities along with the above said infections.
Antibiotic usage and outcomes: Analysis of the case sheets of patients from hospitals revealed that, patients who failed to achieve clinical success or any improvement with different classes of antibiotics were given either meropenem or AAE along with colistin as a combination therapy. Among all the cases analysed, 115 patients received either AAE or meropenem along with colistin and fulfilled above mentioned inclusion criteria were included for this analysis. Antibiotic doses used in the therapy were 3g/ 12 hours, 1g/ 8 hours for AAE and meropenem respectively. For colistin therapy, a loading dose of 9 MIU followed by BD doses of 4.5 MIU were used. Along with these baseline and demographic characteristics, the year-wise usage of both combinations and their respective clinical outcomes were recorded in order to assess the trend in clinical efficacy and resistance incidences over the analysed period. Progress of the therapy was measured in terms of clinical improvement in signs and symptoms.
Patient evaluations and definitions: Clinical parameters of all the selected patients treated during the study period were thoroughly evaluated by examining chest X-rays, culture sensitivity reports (blood, endo-tracheal cultures, Broncho-Alveolar Lavage (BAL) specimens), haematology and biochemistry and other relevant investigations on case to case basis. All the investigations carried throughout the study period were evaluated to correlate the clinical improvement of patient compared to baseline. All the evaluation was done to derive a co-relation of clinical results with clinical parameters. The clinical parameters were also evaluated to rule out any toxicity like nephrotoxicity during course of treatment.
Clinical success: Patient’s response was considered as clinical success, when the patient recovered with either first line empiric antibiotic therapy or a step down from the initial therapy [18].
Clinical failure: An individual case was defined as clinical failure, when either the treatment was switched to other antibiotics (other than AAE, meropenem, colistin).
Results
Among all the patients analysed, 115 patients (n) were given either AAE + colistin (n=52) or meropenem + colistin (n=63). As it was retrospective study and we analysed the case record of only those patients registered between study period, hence the number of patients were unequal in the groups. Baseline and demographic characteristics of these 115 patients is given in [Table/Fig-1] and in most cases, the parameters were comparable among both the groups. Male population was more when compared to their counter parts in both the groups. The mean age of patients in AAE group was 63.65±9.87 years and the same in patients belonging to meropenem group was 61.98±7.81 years. Analysis of disease severity data in terms of APACHE II score and data of different types of infections of both groups are shown in [Table/Fig-1]. Chronic Obstructive Pulmonary Disease (COPD) was the most common co-morbidity observed in patients from both the groups [Table/Fig-2]. Vital parameters recorded for patients in both the groups were largely comparable and are depicted in [Table/Fig-3].
Demographic characteristics of the patients treated during the study period.
| Characteristic | Treatment Groups |
|---|
| AAE + Colistin | Meropenem + Colistin |
|---|
| Evaluable patients (n) | 52 | 63 |
| Sex ratio – male:female [n (%)] | 37:22 (62.71 % : 37.29 %) | 32:22 (59.25 %: 40.75 %) |
| Age (mean±SD) years | 63.65±9.87 | 61.98±7.81 |
| APACHE II score |
| <15 | 16 (30.76 %) | 24 (38.09 %) |
| ≥15 | 36 (69.23 %) | 39 (61.90 %) |
| Type of infection (%) |
| Health Care Associated Pneumonia (HCAP) | 16 (30.76 %) | 21 (33.33 %) |
| Hospital Acquired Pneumonia (HAP) | 21 (40.38%) | 25 (39.68 %) |
| Bloodstream Infections (BSIs) | 11 (21.15 %) | 13 (20.63 %) |
| Ventilator Associated Pneumonia (VAP) | 04 (7.69 %) | 04 (6.34 %) |
| Pathogen family |
| Enterobacteriaceae | 36 (69.23 %) | 42 (66.66 %) |
| non-Enterobacteriaceae | 16 (30.77 %) | 21 (33.34 %) |
Footnotes: SD- Standard Deviation; AAE - Antibiotic Adjuvant Entity
Co-morbidities and laboratory parameters of patients treated in AAE and meropenem groups.
| Number of patients (n) |
|---|
| AAE Group (n = 52) | Meropenem Group (n = 63) |
|---|
| Co-morbidities |
| Coronary artery disease (CAD) | 12 (23.07 %) | 10 (15.87 %) |
| Chronic obstructive pulmonary disease (COPD) | 30 (57.69 %) | 34 (53.96 %) |
| Chronic kidney disease (CKD) | 13 (25.00 %) | 17 (26.98 %) |
| Diabetes mellitus | 23 (44.23 %) | 26 (41.26 %) |
| Cerebrovascular disease | 08 (15.38 %) | 12 (19.47 %) |
| Hypertenstion | 14 (26.92 %) | 11 (17.46 %) |
| Laboratory parameters |
| Arterial pH <7.35 | 14 (26.92 %) | 10 (15.87 %) |
| Blood urea nitrogen level >30 mg/dL (11 mmol/L) | 15 (28.84 %) | 19 (30.15 %) |
| Sodium level <130 mmol/L | 19 (36.53 %) | 24 (38.09 %) |
Vital parameters of patients treated in AAE and meropenem groups.
| Vital parameters | AAE Group(Mean±SD) | Meropenem Group(Mean±SD) |
|---|
| Systolic blood pressure (mmHg) | 123.75±23.32 | 126.70±23.86 |
| Diastolic blood pressure (mmHg) | 68.18±12.90 | 70.38±12.28 |
| Pulse rates (bpm) | 89.45±24.07 | 97.73±22.34 |
| Body temperature (0C) | 38.32±7.91 | 37.96±6.88 |
| Respiration rate (/min) | 21.22±7.57 | 20.88±7.77 |
| Hemoglobin (g/dl) | 10.05±2.05 | 9.98±1.65 |
| Platelet count (/mL) | 220210±126055.38 | 217467.74±105264.80 |
| Total leukocyte count (/mm3) | 15897.30±12662.43 | 16123.17±12162.09 |
| Neutrophils (%) | 80.13±10.02 | 84.88±9.11 |
| Lymphocytes (%) | 10.154±5.84 | 9.13±5.93 |
| Monocytes (%) | 9.81±4.46 | 7.36±4.33 |
| Eosinophils (%) | 1.682±2.34 | 1.37±1.94 |
| Basophils (%) | 0.163±0.20 | 0.140±0.190 |
Footnotes: SD-Standard Deviation
Prior antibiotic therapies given: The patients from both the groups were given a wide range of antibiotics prior to admitting to the Jawaharlal Nehru Medical College, Aligarh. The classes of the prior antibiotics used in patients are given in [Table/Fig-4].
Prior antibiotic therapies given to patients before admitting to ICU.
| Antibiotic Class | AAE GroupN (%) | Meropenem GroupN (%) |
|---|
| Penicillins | 16 (30.76) | 23 (36.50) |
| 3rd gen cephalosporins | 14 (26.92) | 19 (30.15) |
| 4th gen cephalosporins | 09 (17.30) | 07 (11.11) |
| Carbapenems | 29 (55.76) | 37 (58.73) |
| Quinolones | 12 (23.07) | 16 (25.39) |
| Aminoglycosides | 07 (13.46) | 06 (09.52) |
| Penicillin + β lactamase inhibitor | 24 (46.15) | 31 (49.20) |
| Cephalosporins + Aminoglycosides | 20 (38.46) | 27 (42.85) |
| Carbapenms + Aminoglycosides | 38 (73.07) | 41 (65.07) |
| Penicillin + β lactamase inhibitor + Aminoglycosides | 28 (53.84) | 25 (39.68) |
Note – Antibiotics of different classes used.
Penicillins- Amoxicillin, Piperacillin; 3rd gen cephalosporins - Ceftriaxone, Cefoperazone, Ceftazidime; 4th gen cephalosporins – cefepime; Carbapenems – Meropenem, Imipenem, Ertapenem; Quinolones – Ciprofloxacin, Ofloxacin, Levofloxacin; Aminoglycosides - Amikacin, Penicillin + β lactamase inhibitor – Amoxicillin + Clavulanate, Piperacillin + Tazobactam; Cephalosporins + Aminoglycosides - ceftriaxone + Amikacin, Cefoperazone + Amikacin; Carbapenems + Aminoglycosides – Ertapenem + Amikacin, Imipenem + Amikacin, Meropenem + Amikacin; Penicillin + β lactamase inhibitor + Aminoglycosides - Piperacillin + Tazobactam+ Amikacin
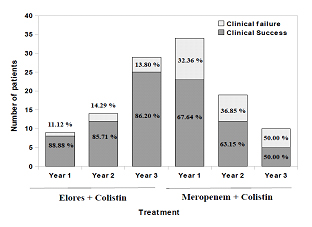
Year-wise usage and clinical response of AAE and meropenem groups: A careful analysis of clinical case sheets of all the patients and segmenting the year-wise AAE and meropenem usage data along with colistin demonstrates the rising trend of AAE sensitivity and declining trend of meropenem usage. Conversely, the clinical cure rates of meropenem group patients decreased every year over a three year period of observation and that of the AAE remained same. Meropenem + colistin therapy was given in 34, 19 and 10 patients admitted in year 1 (November 2012 to October 2013), year 2 (November 2013 to October 2014) and year 3 (November 2014 to October 2015) respectively. Among these, 23 (67.64%), 12 (63.15%) and 05 (50.00%) patients achieved clinical success for respective years with this therapy. On the other hand, AAE + colistin therapy was given in 9, 14 and 29 patients, out of which 08 (88.88%), 12 (85.71%) and 25 (86.20%) patients achieved clinical success for year 1, 2 and 3, respectively [Table/Fig-5]. Patients who failed to respond to meropenem + colistin therapy were shifted to AAE + colistin therapy. In the first year considered for the study, 11 patients were shifted out of which 07 (63.67%) patients achieved clinical success. Similar trend of success rates were followed for the subsequent years [Table/Fig-6].
Year-wise usage of Elores and Meropenem with colistin combination and their respective clinical success.
Note: Year 1, November 2012 – October 2013; Year 2, November 2013 – October 2014; Year 3, November 2014 – October 2015
Clinical success of Meropenem failed patients with Elores therapy.
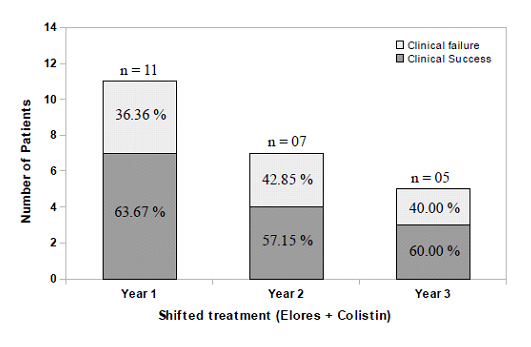
Overall clinical response along with success among the subgroups is depicted in [Table/Fig-7]. Clinical success rate was higher in AAE combination therapy group (86.53%) compared to meropenem combination therapy (63.49%). Clinical response in all the sub groups followed a similar pattern as that of overall clinical response analysed over a period of 3 years. Contrary to the overall clinical success rates the patients which failed to respond to meropenem group had a significantly higher cure rates after their therapy had been shifted to AAE + colistin. The overall success rates with AAE + colistin therapy in these shifted patients was 60.86%.
Clinical success rates among the treatment groups.
| Sub group | Success rate {no. of successes/total no. (%)} for: |
|---|
| AAE + Colistingroup | Meropenem + Colistin group |
|---|
| AAE + Colistin | Meropenem +Colistin | Shifted toAAE + Colistin |
|---|
| Evaluable patients for efficacy analysis | 52 | 63 |
| Overall clinical success | 45/52 (86.53) | 40/63 (63.49) |
| Treatment regime-wise | - | 40/63(63.49) | 14/23 (60.86) |
| Health Care Associated Pneumonia (HCAP) | 14/16 (87.50) | 14/21 (66.66) | 05/07 (71.42) |
| Hospital Associated Pneumonia (HAP) | 19/21 (90.47) | 15/25 (60.00) | 06/10 (60.00) |
| Bloodstream infections (BSIs) | 09/11 (81.81) | 09/13 (69.23) | 02/04 (50.00) |
| Ventilator Associated Pneumonia (VAP) | 03/04 (75.00) | 02/04 (50.00) | 01/02 (50.00) |
| APACHE II score |
| <15 | 15/16 (93.75) | 17/24 (70.83) | 04/07 (57.14) |
| ≥15 | 30/36 (83.33) | 23/39 (58.97) | 10/16 (62.50) |
| Co-morbidities |
| Coronary artery disease (CAD) | 9/12 (75.00 %) | 4/10 (40.00 %) | 04/06 (66.66 %) |
| Chronic obstructive pulmonary disease (COPD) | 26/30 (86.66 %) | 19/34 (55.82 %) | 13/15 (86.66 %) |
| Chronic kidney disease (CKD) | 09/13 (69.23 %) | 08/17 (47.05 %) | 06/09 (66.66 %) |
| Diabetes mellitus | 18/23 (78.26 %) | 14/26 (53.84 %) | 10/12 (83.33 %) |
| Cerebrovascular disease | 06/08 (75.00 %) | 05/12 (41.66 %) | 04/07 (57.14 %) |
| Hypertension | 10/14 (71.42 %) | 05/11 (45.45 %) | 05/06 (83.33 %) |
Discussion
β-lactam antibiotics are the most widely prescribed ones in both community and nosocomial infections [19]. Use of these agents for a long duration, has however resulted in a dramatic increase in the rates of resistance that now threatens the utility of majority of the large drug family. The main mechanism responsible for this resistance is the emergence of β-lactamase enzymes having potent hydrolytic activity against penicillins, cephalosporins and cephamycins [20,21]. Carbapenems are antimicrobial agents that are relatively resistant to hydrolysis by most β-lactamases including Amp-C and have been considered as the last resort drugs all over the world for management of serious infections [22,23]. However, increasing carbapenem resistance among Gram-negative bacteria has been documented greatly in recent years [10,24–26]. To combat increasing carbapenem resistance, several combinations including β-lactam and β lactamase inhibitor combinations (BL + BLI) have received much attention as a cephalosporin alternative drugs in recent past [27–30]. However, over the years there has been considerable increase in the β-lactam + β-lactamase inhibitor (BL + BLI) resistance cases [31,32]. Therefore, new therapeutic options are needed for patients with severe Multi-Drug-Resistant (MDR) infections in whom most classes of antibacterials failed to work. Present study retrospectively analyses data sheets of 115 patients admitted to Jawaharlal Nehru Medical College, Aligarh centre with different infections like HCAP, HAP, VAP and BSIs and treated with either AAE or meropenem in combination with colistin. LRTIs in the considered population, represented most common reason for the admission to the hospital. This is in agreement with the study performed by earlier researchers [33,34].
Analysis of the data sheet revealed that antibiotics belonging to different groups have been used for the treatment of patients. It is a well known fact that inappropriate antimicrobial therapy appear to play an important role in antimicrobial resistance development [35]. The development and spread of antibiotic resistant bacteria are common in ICUs mainly because of heavy use of antibiotics and poor immunity [36,37]. Failure of the patients to respond to such vast groups of antibacterials in the presently studied population, backed by microbial susceptibility data clearly categorizes these infections as multi-drug resistant bacterial infections. It is also known that isolates producing β-lactamases enzymes are also resistant to various groups of antibiotics such as fluoroquinolones, aminoglycosides, tetracyclines and co-trimoxazoles [38,39].
A significantly higher clinical cure data with AAE combination therapy may be attributed to the different ways through which AAE target various resistance mechanism in bacteria such as inhibition of conjugal spreading of resistant gene from one bacteria to another by chelating Mg2+ ions required for the activity of relaxases and thereby inhibiting conjugation process [40], down-regulation of expression of MexAB-OprM and AcrAB-tolC efflux pumps [41]. Synergy is contributed by all components where sulbactam prevents inactivation of ceftriaxone by irreversibly binding to β- lactamases, adjuvant present in AAE chelates the divalent ions (Zn2+) required activity of MBLs and thus AAE deactivates MBLs activity which in turn increase activity of AAE towards microorganisms [42]. Further, AAE is believed to disorganize the EPS and make the cell wall more porous, thus enhancing its entry into the bacterial cells. It has also been found to inhibit curli formation and bacterial adhesion [43]. Treatment failure in 7 (13.47%) patients with AAE + colistin therapy may be attributed to the use of other inappropriate antibacterials empirically, where AAE could have been worked. In support to this, previous studies have demonstrated that, inappropriate therapy, even if corrected at later stages of the treatment led to a clinical failure and mortality. The results of the present study slightly vary from the previous study by Chytra et al., who reported similar clinical cure rates (74.30%) in critically ill patients by meropenem [44]. This study highlights the importance of combination therapy over mono-therapy in critically ill multiple complication cases infected with MDR Gram-negative pathogens in ICUs. Further, a rise in clinical cure rate year by year due to shift over and empiric usage of AAE + colistin therapy is justifiable and may be attributed to the increase in meropenem resistance/ failure cases over the years.
Conclusion
The present study institution experiences a heavy load of MDR Gram-negative infections in critically ill patients with multiple co-morbidities which in turn makes the treatment a challenge. Choice of combination antibiotic such as AAE + colistin therapy is preferred and had higher efficacy as compared to meropenem + colistin and hence score over use of individual antibiotic empirically in hospitals. The study strongly advocates the use of appropriate empiric combination therapy in ICUs not only to achieve higher clinical cure rates but also to lower spread of resistance and associated mortalities. The AAE + colistin therapy has shown better bacteriological and clinical efficacy as compared to meropenem + colistin in the management of various nosocomial MDR Gram-negative infections. A significant number of meropenem failure patients responded to the AAE therapy, highlighting the new hope and way to spare carbapenems and to bring down the growing resistance of carbapenems.
Footnotes: SD- Standard Deviation; AAE - Antibiotic Adjuvant Entity
Footnotes: SD-Standard Deviation
Note – Antibiotics of different classes used.
Penicillins- Amoxicillin, Piperacillin; 3rd gen cephalosporins - Ceftriaxone, Cefoperazone, Ceftazidime; 4th gen cephalosporins – cefepime; Carbapenems – Meropenem, Imipenem, Ertapenem; Quinolones – Ciprofloxacin, Ofloxacin, Levofloxacin; Aminoglycosides - Amikacin, Penicillin + β lactamase inhibitor – Amoxicillin + Clavulanate, Piperacillin + Tazobactam; Cephalosporins + Aminoglycosides - ceftriaxone + Amikacin, Cefoperazone + Amikacin; Carbapenems + Aminoglycosides – Ertapenem + Amikacin, Imipenem + Amikacin, Meropenem + Amikacin; Penicillin + β lactamase inhibitor + Aminoglycosides - Piperacillin + Tazobactam+ Amikacin
[1]. Wenzel RP, Health Care–Associated Infections: Major Issues in the Early Years of the 21st Century 2007 45:S85-88. [Google Scholar]
[2]. Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, Multistate point-prevalence survey of health care–associated infectionsN Engl J Med 2014 370:1198-208. [Google Scholar]
[3]. Manyahi J, Matee MI, Majigo M, Moyo S, Mshana SE, Lyamuya EF, Predominance of multi-drug resistant bacterial pathogens causing surgical site infections in Muhimbili National Hospital, TanzaniaBMC Res Notes 2014 7:500 [Google Scholar]
[4]. Cleven BEE, Palka-Santini M, Gielen J, Meembor S, Kronke M, Krut O, Identification and characterization of bacterial pathogens causing bloodstream infections by DNA microarrayJ Clin Microbiol 2006 44:2389-97. [Google Scholar]
[5]. World Health Organization: The 10 leading causes of death by broad income group (2008) [Google Scholar]
[6]. Reimer LG, Wilson ML, Weinstein MP, Update on detection of bacteremia and fungemiaClin Microbiol Rev 1997 10:444-65. [Google Scholar]
[7]. Horvath DJ, Dabdoub SM, Li B, VanderBrink BA, Justice SS, New paradigms of urinary tract infections: implications for patient managementIndian J Urol 2012 28:154-58. [Google Scholar]
[8]. Minardi D, d’Anzeo G, Cantoro D, Conti A, Muzzonigro G, Urinary tract infections in women: etiology and treatment optionsInt J Gen Med 2011 4:333-43. [Google Scholar]
[9]. Khajuria A, Kumar A, Praharaj Kumar M, Grover N, Carbapenem resistance among Enterobacter Species in a Tertiary Care Hospital in Central IndiaChemother Res Pract 2014 2014:972646 [Google Scholar]
[10]. Hu F, Chen S, Xu X, Guo Y, Liu Y, Zhu D, Emergence of carbapenem-resistant clinical Enterobacteriaceae isolates from a teaching hospital in Shanghai. ChinaJ Med Microbiol 2012 61:132-36. [Google Scholar]
[11]. Cornaglia G, Rossolini GM, The emerging threat of acquired carbapenemases in Gram-negative bacteriaClin Microbiol Infect 2010 16:99-101. [Google Scholar]
[12]. Jalalpour S, Ebadi AG, Role of nano structure of crystalline layer and β- lactamase nano enzyme in antibiotics resistant bacteriaAfr J Pharm Pharmacol 2012 6:113-18. [Google Scholar]
[13]. Rodrigues C, Carbapenem-resistant Enterobacteriaceae: a reality checkRegional Health Forum 2011 15:83-86. [Google Scholar]
[14]. Toolkit CRE, CDC-Guidance for Control of Carbapenem-resistant Enterobacteriaceae (CRE), 2012. http://www.cdc.gov/hai/organisms/cre/cre-toolkit/f-level-prevention.html [Google Scholar]
[15]. Goel N, Wattal C, Oberoi JK, Raveendran R, Datta S, Prasad KJ, Trend analysis of antimicrobial consumption and development of resistance in non-fermenters in a tertiary care hospital in Delhi, IndiaJ Antimicrob Chemother 2011 66:1625-301. [Google Scholar]
[16]. Chaudhary M, Payasi A, A randomidez, open label prospective, multicenter phase-III clinical trial of Elores in lower respiratory tract and urinary tract infectionsJ Pharm Res 2013a 6:409-14. [Google Scholar]
[17]. Chaudhary M, Payasi A, Clinical, microbial efficacy and tolerability of Elores, a novel antibiotic adjuvant entity in ESBL producing pathogens: prospective randomized controlled clinical trialJ Pharm Res 2013b 6:275-80. [Google Scholar]
[18]. Dalfine L, Bruno F, Colizza S, Concia E, Novelli A, Rebecchi F, Cost of care and antibiotic prescribing attitudes for community-acquired complicated intra abdominal infections in Italy: a retrospective studyWorld J Emerg Surg 2014 9:39 [Google Scholar]
[19]. Fritsche TR, Stilwell MG, Jones RN, Antimicrobial activity of doripenem (S-4661): a global surveillance report (2003)Clin Microbiol Infect 2005 11:974-84. [Google Scholar]
[20]. Bush K, The impact of β-lactamases on the development of novel antimicrobial agentsCurr Opin Investig Drugs 2002 3:1284-90. [Google Scholar]
[21]. Villegas MV, Lolans K, Correa A, Suarez CJ, Lopez JA, Vallejo M, First detection of the plasmid-mediated class A carbapenemase KPC-2 in clinical isolates of Klebsiella pneumoniae from South AmericaAntimicrob Agents Chemother 2006 50:2880-82. [Google Scholar]
[22]. Zavascki AP, Carvalhaes CG, Picao RC, Gales AC, Multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii: resistance mechanisms and implications for therapyExpert Rev Anti Infect Ther 2010 8:71-93. [Google Scholar]
[23]. Amjad A, Mirza IA, Abbasi SA, Farwa U, Malik N, Zia F, Modified Hodge test: a simple and effective test for detection of carbapenemase productionIran J Microbiol 2011 3:189-93. [Google Scholar]
[24]. Muthusamy D, Boppe A, Phenotypic methods for the detection of various βlactamases in carbapenem resistant isolates of Acinetobacter baumanii at a Tertiary Care Hospital in South IndiaJ Clin Diagn Res 2012 6:970-73. [Google Scholar]
[25]. Francis RO, Wu F, Della-Letta P, Shi J, Whittier S, Rapid detection of Klebsiella pneumoniae carbapenemase genes in Enterobacteriaceae directly from blood culture bottles by real-time PCRAm J Clin Path 2012 137:527-32. [Google Scholar]
[26]. Grundmann H, Livermore DM, Giske CG, Canton R, Rossolini GM, Campos J, Carbapenem-non-susceptible Enterobacteriaceae in Europe: conclusions from a meeting of national expertsEuro Surveill 2010 15:46 [Google Scholar]
[27]. Hodiwala A, Dhoke R, Urhekar AD, Incidence of metallo-β-lactamase producing Pseudomonas, Acinetobacter and Enterobacterial isolates in hospitalised patientsInt J Pharam Biol Sci 2013 3:79-83. [Google Scholar]
[28]. Chakraborty D, Basu S, Das S, A Study on Infections Caused By Metallo Beta Lactamase Producing Gram-negative bacteria in intensive care unit patientsAm J Infect Dis 2010 6:34-39. [Google Scholar]
[29]. Datta S, Wattal C, Goel N, Oberoi JK, Raveendran R, Prasad KJ, A ten year analysis of multi-drug resistant blood stream infections caused by Escherichia coli and Klebsiella pneumoniae in a tertiary care hospitalInd J Med Res 2012 135:907-12. [Google Scholar]
[30]. Rodriguez-Bano J, Navarro MD, Retamar P, Picon E, Pascual A, β-Lactam/β-lactam inhibitor combinations for the treatment of bacteremia due to extended-spectrum β-lactamase-producing Escherichia coli: A post hoc analysis of prospective cohortsClin Infect Dis 2012 54:167-74. [Google Scholar]
[31]. Meybeck A, Ricard JD, Barnaud G, Eveillard M, Chevrel G, Mounier R, Incidence and impact on clinical outcome of infections with piperacillin/tazobactam resistant Escherichia coli in ICU: A retrospective studyBMC Infect Dis 2008 8:67 [Google Scholar]
[32]. Mohammedi I, Tigaud S, Tournadre JP, Emergence of piperacillin/ tazobactam-resistant Escherichia coliIntens Care Med 2000 26:1584 [Google Scholar]
[33]. Ding JG, Sun QF, Li KC, Zheng MH, Miao XH, Ni W, Retrospective analysis of nosocomial infections in the intensive care unit of a tertiary hospital in China during 2003 and 2007BMC Infect Dis 2009 9:115 [Google Scholar]
[34]. Mukherjee T, Pramod K, Srinivasan G, Rao MY, Nosocomial infections in Geriatric patients admitted I ICUJ Acad Geriat 2005 1:61-64. [Google Scholar]
[35]. Martin SJ, Ohlinger MJ, Nosocomial Gram-Negative infections” in PSAP: pharmacotherapy self-assessment program 2005 Fifth EditionKansas City, MOAmerican College of Clinical PharmacyCopyright [Google Scholar]
[36]. Flaherty JP, Weinstein RA, Nosocomial infection caused by antibiotic-resistant organisms in the intensive-care unitInfect Control Hosp Epidemiol 1996 17:236-48. [Google Scholar]
[37]. Gold HS, Moellering RC Jr, Antimicrobial-drug resistanceN Engl J Med 1996 335:1445-53. [Google Scholar]
[38]. Muller S, Oesterlein A, Frosh M, Abele-Horn M, Valenza G, Characterization of extended-spectrum β- lactamases and qnr plasmid-mediated quinolone resistance in German isolates of Enterobacter speciesMicrobial Drug Res 2011 17(1):99-103. [Google Scholar]
[39]. Lee CH, Liu JW, Li CC, Chien CC, Tang YF, Su LH, Spread of ISCR1 elements containing blaDHA-1 and multiple antimicrobial resistance genes leading to increase of flomoxef resistance in extended- spectrum-β-lactamase-producing Klebsiella pneumoniaeAntimicrob Agents Chemother 2011 55(9):4058-63. [Google Scholar]
[40]. Chaudhary M, Payasi A, Sulbactomax prevents antimicrobial resistance development by inhibition of conjugal transfer of F plasmidsInt J Drug Dev Res 2012 4:337-45. [Google Scholar]
[41]. Chaudhary M, Payasi A, Rising antimicrobial resistance of Pseudomonas aeruginosa isolated from clinical specimens in IndiaJ Proteomics Bioinform 2013 6:5-9. [Google Scholar]
[42]. Chaudhary M, Kumar S, Payasi A, A novel approach to combat acquired multiple resistance in Escherichia coli by using EDTA as efflux pump inhibitorJ Microb Biochem Technol 2012 4:126-30. [Google Scholar]
[43]. Chaudhary M, Kumar S, Payasi A, Role of CSE1034 in Escherichia coli biofilm destructionJ Microb Biochem Technol 2013 5:54-58. [Google Scholar]
[44]. Chytra I, Stepan M, Benes J, Pelnar P, Zidkova A, Bergerova T, Clinical and microbiological efficacy of continuous versus intermittent application of meropenem in critically ill patients: a randomized open-label controlled trialCrit Care 2012 16:113 [Google Scholar]