Leptospirosis is a globally widespread bacterial zoonosis caused by spirochetes belonging to genus Leptospira [1]. It has been classified as an emerging or re-emerging infectious disease by the Centers for Disease Control and Prevention and World Health Organization (WHO) [2,3]. An estimated 500,000 cases occur annually, with fatality range rising up to 70% as mentioned in different cohort study [4]. Leptospirosis disease mainly affects the agricultural farmers and urban slum dwellers as in resources poor developing countries are unable to provide basic medical services in these areas so the WHO considers leptospirosis to be a neglected zoonotic disease [5,6]. Identifying leptospirosis is a diagnostic challenge, because of its protean manifestations which vary from asymptomatic or mild flu like cases to a severe fulminant disease presenting with jaundice, renal failure, pneumonia, haemorrhage and shock [7].
Various diagnostic methods like Rapid tests, IgM dipstick assay, Slide agglutination test, IgM dot ELISA, dipstick test, IgM ELISA, MAT (Microscopic Agglutination Test), indirect hemagglutination assay etc., are available. Besides these diagnostic approaches, development of accurate molecular based detection methods like Polymerase Chain Reaction (PCR) has been a major advance for leptospirosis diagnosis during first week of illness [8].
PCR can be used in conjunction with other diagnostic tests for rapid and accurate diagnosis to initiate proper and timely management [9]. This study was designed with the aim to detect the leptospiral DNA by PCR method in serum of the suspected leptospirosis patients in early phase of illness and to compare the results of PCR with that of rapid leptocheck, IgM ELISA and MAT.
Materials and Methods
Patients and sera: A retrospective study was conducted during the period of July 2008 to November 2008. A total of 207 blood samples were collected from clinically suspected cases of leptospirosis according to WHO case definition [3], “any patient presenting with an abrupt onset of fever, chills, conjunctiva suffusion, headache, myalgia, oliguria, jaundice, breathlessness and haemoptysis” admitted in New Civil Hospital, Surat and from admitted patients of peripheral health centre of South Gujarat. Informed consent was taken from all participants. All patients of 15-55 years of age were included in present study. Patient having other infection like malaria, dengue, hepatitis, typhoid etc., that was diagnosed by laboratory was excluded from the study. Blood samples were centrifuged at 1000g for 10 minutes and separated serum stored at -20°C. The enrolled patients in present study were requested for convalescent sample after 15 days of first sample collection. Both acute and convalescent phase sera were subjected to Rapid Leptocheck, IgM ELISA and MAT. PCR was done only in acute phase (first serum sample) sera. Leptospira culture of freshly collected whole blood samples was done in Ellinghausen McCullough Johnson Harris (EMJH) liquid and semi-solid medium.
Rapid leptocheck test: (Rapid leptocheck WB) 10μL from each sample was tested as per manufacturer’s instruction. It utilizes the principle of immunochromatography, a unique two-site immunoassay on a membrane. As the test sample flow through the membrane of the test device, the anti-human IgM colloidal gold conjugate forms a complex with IgM antibodies in the sample. When this complex moves in the cassette to the test window ‘T’, it is immobilized by the genus specific antigen of leptospira coated on the membrane, which lead to the formation of a red to deep purple coloured band at the test region. If the band is present on ‘T’ region it confirms a positive test result. If there is no band at the test region, it indicates negative result. At the “C” region, the anti-rabbit antibodies are coated. If any unreacted conjugate and the unbound complex present in the sample, it will move further on the membrane and are subsequently immobilized at “C” window forming a red to deep purple coloured band. If there is no control band formed at this region, it suggests the test is invalid [10].
IgM ELISA test: All serum samples were evaluated using IgM ELISA (Institute Virion, SerionGmbh, Wurzhurg Germany) as per the manufacturer’s instruction [11]. At each test run, laboratory had kept sets of a positive control, negative control and cut-off calibrator in duplicate.
Calculation for Serion ELISA classic leptospira IgM: Serion units of < 40 were interpreted as a negative result and ≥ 40 was interpreted as a positive result.
Microscopic Agglutination test: The MAT test was performed using standard procedure. MAT test includes different serogroups which were used as an antigen in each test run. The following serogroups were used in the test: L. australis (Australis), L. autumnalis (Bangkinang), L. ballum (Ballum), L. sejroe (Hardjo), L. canicola (Canicola), L. hebdomadis (Hebdomadis), L. pomona (Pomona),L. grippotyphosa (Grippotyphosa), L. pyrogen (Pyrogen), L. Icterohaemorrhagiae (Icterohaemorrhagiae),L. semeranga (Patoc1). These strains were obtained from National Leptospirosis Reference Centre, RMRC, WHO collaborating centre in Portblair, Andaman and Nicobar Island. These serovars were maintained in semisolid 0.1% EMJH agar supplemented with 10% enrichment (Difco, USA). These strains were maintained at 28-30°C temperature for incubation.
Preparation of antigen: From the panel of 11 serovars, 0.5ml of each strain was inoculated into 10ml of liquid EMJH. These inoculated test tubes were kept in incubator at 28-30°C for five to seven days. Culture was checked after 5 days of inoculation under dark field microscopy to see that absence of any contamination or clumps or presence of enough quantity of growth which was required in MAT test. A growth should be as appropriate as McFarland standard 1 so density of leptospira should be approximately 2-3 x 108 leptospira /ml of media.
A total of 96 well flat bottomed microtitre plates was used in MAT test. Serum was diluted by using Phosphate Buffer Saline. Dilution of serum was started from 1 in 25 to 1 in 1600. A 50μl of the specific serovar (McFarland 1.0) added to all the wells. One of the wells with antigen only, without addition of antibody served as an antigen control. The final dilution after adding the antigen was 1 in 50 to 1 in 3200. The plate was covered with aluminium foil and incubated at 37°C for 2 hours in wet chamber to avoid dehydration. Slide was examined by dark field microscopy at a magnification of 40X after 2 hours of incubation. The reporting of end point titer was the highest serum dilution which showed approximately 50% agglutinated leptospires or reduction in the number of leptospiral cells as compared to the antigen control. A titer of 1 in 100 or more was considered as a significant titer [12,13]. If any sera showed seroconversion or fourfold rise of antibodies titre in MAT test between acute and convalescent phase serum or positive by IgM ELISA was considered as a confirm case of leptospirosis.
Real time PCR assay: Total DNA from human serum (200μl) was prepared using QIAamp DNA Mini kits (QIAGEN, USA) according to the manufacturer’s instructions. The primers and probes were designed from alignments of available Leptospira spp. LipL32 sequences obtained from the GenBank nucleotide sequence database. Assay used were Taqman® gene expression assay. The program used was Primer Express™ (Applied Biosystems, USA). For Real Time PCR, 8.8μl of DNA was added to the 11.2μl Taqman Universal PCR Master mix (Applied Biosystems, USA). A negative control without added template in the above reaction mixture was used as a control to detect the presence of contaminating DNA. Amplification and fluorescence detection was conducted in an ABI Prism 7300 sequence detector (Applied Biosystems, USA) with a program of 50 cycles, each cycle consisting of initial denaturation at 50 °C for 2 minute, 95°C for 10 minute followed by primer annealing at 95°C for 15 seconds and extension at 60°C for one minute as per the manufacturer’s instructions. The oligonucleotide primers used were 5’-GGATCTGTGATCAACTATTAC-3’, reverse primer 3’-CGAACTCCCATTTCAGCGATTAC-5’ and reporter 1 sequence was TCCGGCGCTTGTCCTG with reporter 1 dye FAM and reporter 1 quencher NFQ.
Blood culture: Whole blood samples collected upon admission of patients were cultured in EMJH liquid medium supplemented with enrichment medium and 5-fluorouracil (200μg/ml) and incubated aerobically at 28°-30°C. A drop from each culture medium was checked weekly by dark field microscopy from second week onwards and kept for 4 months before being discarded as negative [14].
Results
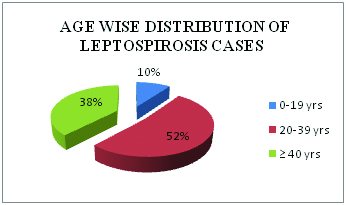
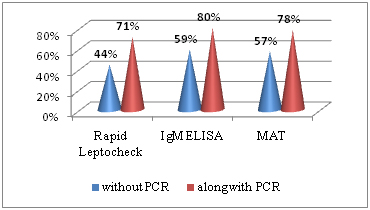
Total 207 patients with clinically suspected cases of leptospirosis were enrolled in present study. Cases were reported between July to November month during the year 2008. An adult age group from 20-39 year was more commonly infected by these leptospira [Table/Fig-1]. Gender distribution of leptospirosis shows 169 (82%) cases, males were affected. Seropositivity rate of different laboratory test were shown in [Table/Fig-2], 91(44%) cases were detected by Rapid leptocheck test in early phase of disease, along with PCR detection rate reached to 146 (71%). Seropositive cases by IgM ELISA alone were 122 (59%) which increased to 165 (80%) with PCR. By MAT, seropositivity was found in 118 (57%) cases if MAT test was done alone but combination with PCR seropositivity of MAT was increased upto 78% [Table/Fig-3]. Comparison of PCR with other tests considering MAT as gold standard was shown in [Table/Fig-4]. Sensitivity, specificity and accuracy of PCR were 52%, 79% and 57% respectively. No leptospira in culture was isolated.
Age wise distribution of leptospirosis cases.
Seropositivity rate of different laboratory tests.
| Stage of disease | Seropositivity by Rapid Leptocheck test (%) | Seropositivity by IgM ELISA (%) | Seropositivity by MAT (%) |
|---|
| Acute phase | 91 (44%) | 122 (59%) | 118 (57%) |
| Convalescent phase | 159 (77%) | 161 (78%) | 161 (78%) |
Showing the increase detection rate seropositive cases by different serological tests along with PCR in acute phase of leptospirosis illness.
Comparison of PCR with other diagnostic tests considering MAT as gold standard in acute phase of leptospirosis disease.
| PCR (%) | Rapid test (%) | ELISA (%) |
|---|
| Sensitivity | 52 | 65 | 84 |
| Specificity | 79 | 83 | 75 |
| PPV | 90 | 83 | 81 |
| NPV | 30 | 63 | 78 |
PPV - Positive Predictive Value
NPV - Negative Predictive Value
Discussion
Leptospirosis is widely recognized as acute febrile disease and being emergent or re-emergent in tropical and sub-tropical regions. Leptospirosis is frequently under-diagnosed, also challenging as culture of Leptospira and seroconversion require weeks [15]. Isolation of Leptospira from the clinical specimen is difficult because leptospires are fastidious, slow growing that requires special growth media and it is time consuming and laborious [16,17]. Therefore, PCR assay is highly useful as a contemporary method for diagnosis in acute phase of leptospirosis.
Many previous studies had mentioned the higher sensitivity of PCR when samples are taken early and before initiation of anti-microbial therapy [18–20] and showed that PCR increases the detection rate in early phase of disease. Leptospira disseminates to most tissues and body fluids like blood, spinal fluid in acute phase, where they multiply rapidly [21–23]. Moreover, PCR positivity peaked from 4th day to 8th day after onset of symptoms; even cases were detected upto 15 days without any significant difference in sensitivity of assay [24].
In leptospirosis, antibodies begin to appear within a few days of onset of symptoms and in a significant proportion of patients the antibodies persist in detectable quantities for several months. Various rapid serologic tests are available, but these tests have low sensitivity in early acute phase. They are primarily IgM detection tests which are not detectable till 5-7 days of onset of symptoms [9,16]. In present study, 91(44%) suspected cases were positive by rapid leptocheck serological test in early phase of disease, while PCR test had detected 55 (27%) more cases which would not have been detected by Leptocheck alone. Considering ELISA, 59% suspected cases were positive by IgM ELISA; however it was 80 % with PCR. Similarly, positivity of MAT increases from 57% to 78% with PCR. Since, IgM ELISA and MAT detects early antibodies, they are the most common diagnostic approach to leptospirosis. MAT is a gold standard method but, titers are usually low during the acute stage of the disease, therefore diagnosis is difficult on basis of single serum sample. MAT test is also technically challenging and requires maintenance of live Leptospira strains including local prevalent strains.
The gold standard MAT analysis of present study was compared with other studies [Table/Fig-5]. In these studies sensitivity of IgM ELISA ranges from 43% to 100% and specificity ranges from 76% to 98%. Leptocheck WB sensitivity ranges from 78% to 93% and specificity ranges from 84% to 98%. Sensitivity and specificity of PCR in Fonseca et al., was 62% and 100%, but in Smita shekatkar et al., it was 11% and 95% respectively. These all results show a correlation with present study [9,11,13,14,24–26].
Comparison with other study results showing sensitivity and specificity of rapid Leptochek WB, IgM ELISA and PCR [9,11,13,14,24–26].
| References | Sensitivity (%) | Specificity (%) |
|---|
| Rapid Leptocheck WB test | MG Goris [14] | 78 | 98 |
| Niloofa R et al., [24] | 86 | 84 |
| Panwala et al., [11] | 93 | 86 |
| Present study | 65 | 83 |
| ELISA | Kucerova et al., [25] | 100 | 88 |
| Effler et al., [9] | 48 | 98 |
| Fonscea et al., [26] | 79 | 89 |
| Smita Shekatkar [13] | 43 | 76 |
| Present study | 84 | 75 |
| PCR | Fonscea et al., [26] | 62 | 100 |
| Smita Shekatkar [13] | 11 | 95 |
| Present study | 52 | 79 |
Fonseca et al., showed that sensitivity increases to 96.5% in IgM ELISA and 93.1% in MAT along with PCR [26]. Ooteman M et al., detected 13-29% positive cases by PCR among 45 unconfirmed cases by MAT [27]. Biswas D et al., showed the sensitivity of single MAT at diagnostic titre of 1 in 80 with PCR, were found to be 56 % [28]. The efficacy of other commonly used test was estimated by comparing the results of these tests with PCR results considering MAT as gold standard.
Currently, molecular based detection methods have redefined the reference standard because their sensitivity was more than other diagnostic tests and they can even detect low levels of leptospires in acute phase of illness [24,29]. From the clinical point of view, the ability to detect the infection early in the course of the disease is of extreme importance for initiating appropriate treatment to avoid serious complications.
Limitation
The present study has a limitation that Leptospira isolates were not obtained. Reason could be the delay of sample collection after the onset of symptoms. Patients referred from peripheral health center had already taken primary treatment which in turn decreases the chances of leptospira isolation from blood. Although PCR was less sensitive than serological reactions throughout the course of the illness, may give erroneous results when inhibitory factors are present in the samples that impairs the amplification process [30] as well as when leptospires are present in very low numbers. PCR detects DNA while other tests detect antibody in sera so, PCR is an effective complementary test in the first phase of the disease, particularly when no specific antibodies were detected in serological reaction and helps in detection of more cases which would have been missed by antibody test [31]. A limitation of PCR based diagnosis of leptospirosis is the inability of the assays to identify the infecting circulating serovars in particular geographical area [18,32].
Conclusion
PCR is very sensitive and specific test in the acute phase of illness, it picked up to 50% of cases which were negative by other serological tests. This result showed that the PCR method has advantages over other serological test in the early diagnosis of Leptospirosis. The identity of infecting serovars has significant epidemiological and public health measures but the prompt diagnosis of Leptospirosis is essential for both patients care and efficient implementation of public health measures, so use of PCR with other diagnostic methods in early phase of leptospirosis increases the detection rate.
PPV - Positive Predictive Value
NPV - Negative Predictive Value