Motor Nerve Conduction Velocity In Postmenopausal Women with Peripheral Neuropathy
Akanksha Singh1, Naiyer Asif2, Paras Nath Singh3, Mohd Mobarak Hossain4
1 Senior Resident, Department of Physiology, All India Institute of Medical Sciences, New Delhi, India.
2 Professor, Department of Orthopaedics, J.N. Medical College, Aligarh Muslim University, Aligarh, Uttar Pradesh, India.
3 Professor and Head, Department of Physiology, G S Medical College, Hapur, Uttar Pradesh, India.
4 Professor, Department of Physiology, J.N. Medical College, Aligarh Muslim University, Aligarh, Uttar Pradesh, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Akanksha Singh, Senior Resident, Department of Physiology, Second Floor, Teaching Block All India Institute of Medical Sciences, Ansari Nagar, New Delhi-110029, India.
E-mail: drakanksha111@gmail.com
Introduction
The post-menopausal phase is characterized by a decline in the serum oestrogen and progesterone levels. This phase is also associated with higher incidence of peripheral neuropathy.
Aim
To explore the relationship between the peripheral motor nerve status and serum oestrogen and progesterone levels through assessment of Motor Nerve Conduction Velocity (MNCV) in post-menopausal women with peripheral neuropathy.
Materials and Methods
This cross-sectional study was conducted at Jawaharlal Nehru Medical College during 2011-2013. The study included 30 post-menopausal women with peripheral neuropathy (age: 51.4±7.9) and 30 post-menopausal women without peripheral neuropathy (control) (age: 52.5±4.9). They were compared for MNCV in median, ulnar and common peroneal nerves and serum levels of oestrogen and progesterone estimated through enzyme immunoassays. To study the relationship between hormone levels and MNCV, a stepwise linear regression analysis was done.
Results
The post-menopausal women with peripheral neuropathy had significantly lower MNCV and serum oestrogen and progesterone levels as compared to control subjects. Stepwise linear regression analysis showed oestrogen with main effect on MNCV.
Conclusion
The findings of the present study suggest that while the post-menopausal age group is at a greater risk of peripheral neuropathy, it is the decline in the serum estrogen levels which is critical in the development of peripheral neuropathy.
Hormone replacement therapy, Menopause, Oestrogen, Progesterone
Introduction
With the rise in global life expectancy women spend about one third of their life in the post-menopausal period [1]. The menopause is characterised by a new milieu of the ovarian hormones with a decline in the serum oestrogen and progesterone levels [2]. This change is concomitant with effects over multiple systems including an increased risk or exacerbation of osteoporosis, cardiovascular diseases and autoimmune disorders [3–8]. Effect over the nervous system has also been studied with special emphasis on the role of serum oestrogen and progesterone as these hormones form the basis of the menopause Hormone Replacement Therapy (HRT) [9–11]. The role of oestrogen and progesterone as a neuroprotective hormone has been studied in different clinical and experimental settings indicating a neuroprotective and neurotrophic role of these hormones [12–16]. On the other hand, there are reports which show higher progesterone levels associated with slower nerve conduction [17,18] and inconclusive or absent role of neuroprotective role of oestrogen and progesterone [19,20]. Studies reporting the status of peripheral nervous system in menopause have shown a higher incidence of peripheral neuropathy in women with menopause [21–23]. Kim et al., have evaluated the MNCV in peripheral nerves in post-menopausal women and found lower posterior tibial and median motor nerve conduction velocities in post-menopausal women not receiving HRT as compared to post-menopausal women receiving HRT [24].
Despite evidence for the greater susceptibility of post-menopausal women for peripheral neuropathy, there are only a few studies investigating the condition of peripheral nerves in post-menopausal women and its correlation with the post-menopausal hormone profile [21,24]. Assessment of Motor Nerve Conduction Velocity (MNCV) is a reliable and quantitative method for assessment of the status of fast conducting peripheral motor nerve fibres [25]. The aim of the present study was to explore the probable relationship between the peripheral motor nerve status and hormone levels through assessment of MNCV in post-menopausal women with peripheral neuropathy as compared to control (post-menopausal women without peripheral neuropthy) and its correlation with their serum oestrogen and progesterone levels.
Materials and Methods
This was a cross-sectional study conducted in the Neurophysiology laboratory, in the Department of Physiology in collaboration with Department of Orthopaedics at Jawaharlal Nehru Medical College (JNMC), Aligarh Muslim University, Aligarh, during 2011-13, after approval from the JNMC ethics committee. Thirty post-menopausal women (>1year of cessation of menses) with clinically diagnosed peripheral neuropathy were recruited from the Orthopaedics out-patient department at JNMC, AMU, Aligarh. They were compared with 30 age matched post-menopausal women without peripheral neuropathy. The sample size was decided based on feasibility and operational constraints. Patients with co-morbidities with risk of neuropathies like diabetes, hypertension, malnourishment and exposure to toxic agents etc. were excluded from the study based on history. Informed written consent were obtained from all the subjects after explanation of the type, purpose and duration of the study.
Motor Nerve Conduction Velocity: MNCV was recorded bilate-rally for median, ulnar and Common Peroneal Nerves (CPN) with the help of Electromyography (EMG)/ Nerve conduction velocity (NCV) equipment with Neuroperfect software (Medicaid Systems, Chandigarh, India). Bipolar stimulator was used for providing supramaximal stimulation (determined individually) with square wave pulse of 0.1ms duration. All recordings were done in room with temperature of 26±2°C. The response in the form of Compound Muscle Action Potential (CMAP) was recorded with the help of gold plated surface disc electrodes which were attached after cleaning the recording site with spirit and application of conduction jelly. One active and one reference electrode was placed over recording site and a ground electrode was placed between the stimulation and the recording site. Band pass filter of 2Hz - 5KHz was applied and sweep speed was set at 5ms/division.
For estimation of median MNCV the nerve was stimulated sequentially at wrist (proximal site) and antecuital fossa (distal site) with simultaneous CMAP recording from Abductor Pollicis Brevis (APB) muscle. The MNCV was calculated by measuring the distance between the proximal and distal stimulation sites and dividing it with the difference in the latencies of CMAP in each instance.
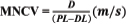
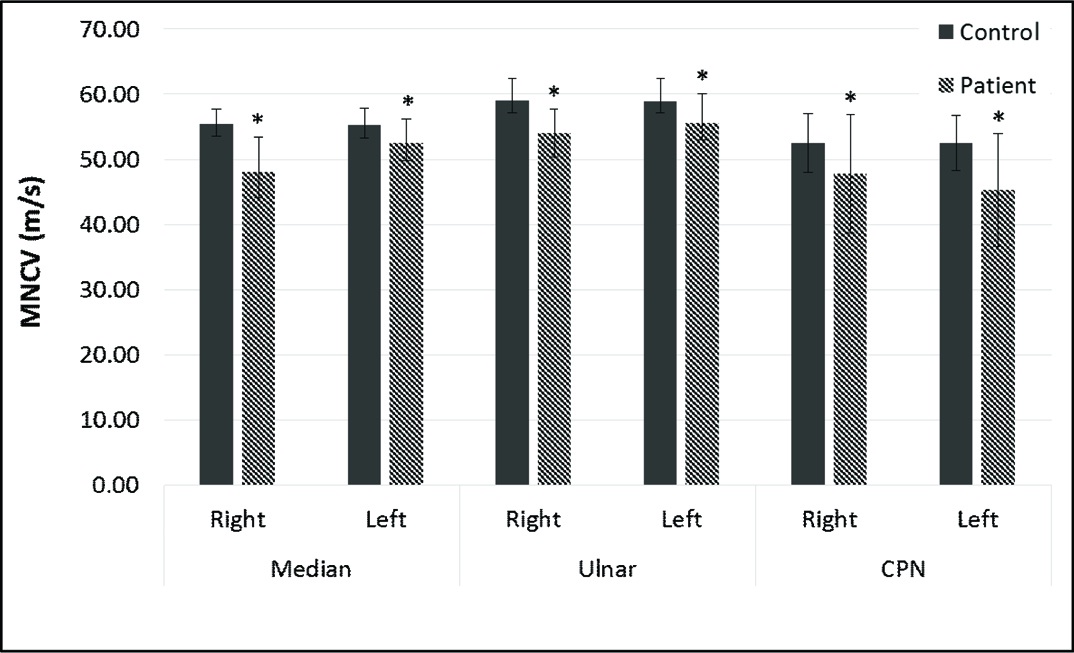
MNCV : Motor Nerve Conduction Velocity
D : Distance between proximal and distal stimulation sites (mm).
PL : Latency on Proximal stimulation (ms).
DL : Latency on Distal stimulation (ms)
Similarly, MNCV was calculated for ulnar and CPN. In case of ulnar nerve the stimulation was done at wrist and elbow with CMAP recorded from Abductor Digiti Minimi (ADM). For CPN the stimulation was provided at ankle and the neck of fibula and CMAP recorded from Extensor Digitorum Brevis (EDB).
Serum Oestrogen and Progesterone and estimation: Blood was collected by venipuncture and allowed to clot. The serum was separated by centrifugation at room temperature and stored at 20C. The serum analysis was done in the Biochemistry department, JNMC, Aligarh. Serum oestrogen was estimated by DRG Estradiol Enzyme-Linked ImmunoSorbent Assay (ELISA) EIA-2693 (DRG International, Inc., USA). Serum progesterone was estimated with XEMA Progesterone EIA-K207 (XEMA Co., Ltd. Clinical Diagnostics, Russia). Both were enzyme immunoassays based on competition enzyme immunoassay principle.
Statistical Analysis
The data was analysed using SPSS software (Version 22, IBM). The normality of distribution was estimated with Shapiro-Wilk test. Quantitative data is expressed as mean±SD for normally distributed data and median with Interquartile Range (IQR) is used to express skewed data. Only age, CPN and MNCV as a parameter showed parametric distribution and were compared with unpaired student t-test while for the rest of the parameters Mann Whitney u-test was applied. The p< 0.05 was considered to be statistically significant. Stepwise linear regression analysis was done with age, serum oestrogen and progesterone as independent variables to determine their relationship with MNCV.
Results
The study was conducted on 30 post-menopausal women with peripheral neuropathy (the patient group) and 30 age matched post-menopausal women without peripheral neuropathy (the control group). A comparison of age distribution and the serum oestrogen and progesterone profile is presented in [Table/Fig-1]. There was no significant difference in the age between the two groups. However, significantly lower serum oestrogen as well as serum progesterone was observed in the patients as compared to the control group.
Age and serum oestrogen and progesterone levels in the control and the patient group.
| Control | Patient | p-value |
|---|
| Age (years)(Mean±SD) | 52.5±4.9 | 51.4±7.9 | 0.534 |
| S. Oestrogen (pg/ml)(Median (IQR) | 33.00 | 22.63* | 0.0001 |
| (25.45- 41.16) | (12.19- 30.43) |
| S. Progesterone (ng/ml){Median (IQR)} | 0.29 | 0.19* | 0.003 |
| (0.22- 0.40) | (0.09-0.31) |
SD: Standard Deviation; IQR; Interquartile range; *significant as compared to the control (p < 0.05). Unpaired student t-test was used for comparison of age. Mann Whitney U test was applied for comparison of Serum oestrogen and progesterone.
A significantly lower MNCV was observed in patients with peripheral neuropathy as compared to the control subjects, bilaterally and in all the nerves tested. The results are presented in [Table/Fig-2].
Motor nerve conduction velocity in the control group and postmenopausal women with peripheral neuropathy (patient).
CPN: Common peroneal nerve; *significant as compared to the control (p < 0.05). Unpaired student t-test was used for comparison of CPN MNCV. Mann-Whitney U test was applied for comparison of Median and Ulnar MNCV.
Stepwise linear regression analysis with age, serum oestrogen and progesterone as the independent variables, showed that serum oestrogen has significant main effect on the motor conduction velocities [Table/Fig-3]. Post-hoc power of the study was calculated with an alpha of 0.05 using the data obtained and it was found to be 81.9%.
Result of stepwise linear regression analysis with serum oestrogen as the independent variable with main effect.
| MNCV | beta | R2 |
|---|
| Median N | Right | .532a* | .284 |
| Left | .424a* | .180 |
| Ulnar N | Right | .420a* | .177 |
| Left | .372a* | .138 |
| CPN | Right | .397a* | .158 |
| Left | .390a* | .152 |
CPN: Common peroneal nerve; *significant (p < 0.05); aadjusted
Discussion
This study was done to evaluate the status of peripheral motor nerve through MNCV in post-menopausal women with peripheral neuropathy and post-menopausal women without peripheral neuropathy and the correlation of the motor nerve conduction velocities with the serum oestrogen and progesterone. As expected, women with peripheral neuropathy had a significantly lower MNCV as compared to the control group. On comparison of the serum oestrogen and progesterone levels, the post-menopausal women with peripheral neuropathy had significantly lower levels of both serum oestrogen and progesterone levels. These results are similar to a previous study by Kim et al., where a lower tibial and median MNCV were observed in post-menopausal women without HRT as compared to those receiving HRT [24].
A few other studies have shown that the post-menopausal women have increased risk of developing peripheral neuropathy suggesting the role of the changed hormonal environment [21–23]. Bjorkqvist et al., studied ovariectomized patients and concluded that carpal tunnel syndrome was common in iatrogenically induced menopause also [23]. Pascual et al., studied oophorectomized women and age matched menstruating women and found greater incidence of carpal tunnel syndrome in menopausal women [21].
To ascertain the relationship between the serum levels of oestrogen and progesterone a stepwise linear regression analysis was done which showed that only serum estrogen level has the significant main effect as an independent variable on the MNCV.
The effect of oestrogen on nervous system has been studied earlier with reports showing an absence of effect on cognitive functions in women in mid and late life [19]. Alzheimer’s disease [26] and healing and neural repair at suture site in rat sciatic nerve [20]. These results however should be analysed in light of the fact that ovaries are not the only source of oestrogen. It is also a neurosteroid synthesized within the nervous system. Thus, serum levels may not always be representative of the actual whole body concentrations [19]. Additionally, there are other reports which show improved cognitive performance in post-menopausal women on treatment with oestrogen [27]. Mice neural stem cell studies have shown neural repair by estradiol through promotion of angiogenesis and endothelial differentiation [14]. Numerous other studies have shown the role of oestrogen in protection and repair of neural tissue both in central as well as peripheral nervous system [12,13].
In the present study the effect of serum progesterone on MNCV was not observed. The effect of progesterone has been investigated previously but the results are variable. Azarmina et al., studied visual evoked potential during menstrual cycle and reported decreased optic nerve conduction velocity with higher progesterone levels [17]. Similarly, it has been shown that elevated progesterone levels during pregnancy are negatively correlated with cholesterol availability which has a negative impact on nerve conduction [18]. Another study has shown that a combination of oestrogen and progesterone to be ineffective in neural repair through prevention of scar tissue formation in rat’s sciatic nerve [20]. However, workers have also reported a neuroprotective role of progesterone on electrophysiological alternation in STZ-induced diabetic neuropathy in rats [16]. Similar neuroprotective effects on NCV were reported in another study on diabetic neuropathy in rats. However, the various effects of progesterone were mediated by different receptors [28]. The neuroprotective role of progesterone thus appears to be variable with the possible role of different receptors in different tissues and species.
Limitation and Perspectives
The limitations of the present study are that we have focused at only a single electrophysiological parameter and the study sample size was not large. Future studies with larger sample size and more extensive neurophysiological parameters would help in confirming the results. Furthermore, the study does not include anthropometric or dietary parameters which could have added to the present observations. A more detailed neurophysiological study with inclusion of dietary and anthropometric parameters, will be more instructive in interpreting the role of oestrogen and progesterone in the peripheral nervous system. The results of present study can help in future formulations of protocols for utilizing simple elctrophysiological tests to identify at risk post-menopausal women and instituting prophylactic HRT harnessing the neuroprotective effect of oestrogen.
Conclusion
The findings of the present study suggest that while the post-menopausal age group is at a greater risk of developing peripheral neuropathy, it is the decline in serum oestrogen level which is critical in the development of peripheral neuropathy. This is in line with earlier reports showing the role of oestrogen in neural protection and regeneration. The role of progesterone still remains unclear.
SD: Standard Deviation; IQR; Interquartile range; *significant as compared to the control (p < 0.05). Unpaired student t-test was used for comparison of age. Mann Whitney U test was applied for comparison of Serum oestrogen and progesterone.
CPN: Common peroneal nerve; *significant (p < 0.05); aadjusted
[1]. World Health Organisation. World Health Statistics [Internet]. 2016. Available from: http://www.who.int/gho/publications/world_health_statistics/EN_WHS08_Full.pdf [Google Scholar]
[2]. Hale GE, Burger HG, Hormonal changes and biomarkers in late reproductive age, menopausal transition and menopauseBest Pract Res Clin Obstet Gynaecol [Internet] 2009 23(1)Elsevier Ltd:7-23. [Google Scholar]
[3]. Hu FB, Grodstein F, Hennekens CH, Colditz G a, Johnson M, Manson JE, Age at natural menopause and risk of cardiovascular diseaseArch Intern Med 1999 159(10):1061-66. [Google Scholar]
[4]. Shuster LT, Rhodes DJ, Gostout BS, Grossardt BR, Rocca WA, Premature menopause or early menopause: long-term health consequencesMaturitas [Internet] 2010 65(2):161-6.Available from: http://www.ncbi.nlm.nih.gov/pubmed/19733988 [Google Scholar]
[5]. Ragonese P, D’Amelio M, Salemi G, Aridon P, Gammino M, Epifanio A, Risk of Parkinson disease in women: effect of reproductive characteristicsNeurology 2004 62(11):2010-14. [Google Scholar]
[6]. Hadjidakis DJ, Kokkinakis EP, Sfakianakis ME, Raptis SA, Bone density patterns after normal and premature menopauseMaturitas 2003 44(4):279-86. [Google Scholar]
[7]. Bove R, Chitnis T, Houtchens M, Menopause in multiple sclerosis: Therapeutic considerationsJ Neurol 2014 261(7):1257-68. [Google Scholar]
[8]. Woods NF, Mitchell ES, Schnall JG, Cray L, Ismail R, Taylor-Swanson L, Effects of herbal preparations on symptoms clusters during the menopausal transitionClimacteric [Internet] 2014 17(1):10-22. [Google Scholar]
[9]. Schumacher M, Guennoun R, Ghoumari A, Massaad C, Robert F, El-Etr M, Novel perspectives for progesterone in hormone replacement therapy, with special reference to the nervous systemEndocr Rev 2007 28(4):387-439. [Google Scholar]
[10]. Tan D, Darmasetiawan S, Haines CJ, Huang K-E, Jaisamram U, Limpaphayom KK, Guidelines for hormone replacement therapy of Asian women during the menopausal transition and thereafterClimacteric [Internet] 2006 9(2):146-51. [Google Scholar]
[11]. Goodman NF, Cobin RH, Ginzburg SB, Katz IA, Woode DE, American Association of Clinical Endocrinologists. American association of clinical endocrinologists medical guidelines for clinical practice for the diagnosis and treatment of menopauseEndocr Pract [Internet] 2011 17(Suppl 6):1-25.Available from: http://www.ncbi.nlm.nih.gov/pubmed/22193047 [Google Scholar]
[12]. Suzuki S, Brown CM, Wise PM, Mechanisms of neuroprotection by oestrogenEndocrine 2006 29(2):209-15. [Google Scholar]
[13]. Wise PM, Dubal DB, Wilson ME, Rau SW, Böttner M, Rosewell KL, Estradiol is a protective factor in the adult and aging brain: Understanding of mechanisms derived from in vivo and in vitro studiesBrain Res Rev 2001 37(1-3):313-19. [Google Scholar]
[14]. Sekiguchi H, Ii M, Jujo K, Thorne T, Ito A, Klyachko E, Estradiol promotes neural stem cell differentiation into endothelial lineage and angiogenesis in injured peripheral nerveAngiogenesis 2013 16(1):45-58. [Google Scholar]
[15]. Sekiguchi H, Ii M, Jujo K, Renault M-A, Thorne T, Clarke T, Estradiol triggers sonic-hedgehog-induced angiogenesis during peripheral nerve regeneration by downregulating hedgehog-interacting proteinLab Invest [Internet] 2012 92(4):532-42. [Google Scholar]
[16]. Sameni HR, Panahi M, Sarkaki A, Saki GH, Makvandi M, The neuroprotective effects of progesterone on experimental diabetic neuropathy in ratsPak J Biol Sci [Internet] 2008 11(16):1994-2000. [Google Scholar]
[17]. Azarmina M, Soheilian M, Azarmina H, Increased latency of visual evoked potentials in healthy women during menstruationJ Ophthalmic Vis Res 2011 6(3):183-86. [Google Scholar]
[18]. Amir D, Fessler DMT, Boots for Achilles: Progesterone’s reduction of cholesterol is a second-order adaptationQ Rev Biol [Internet] 2013 88(2):97-116. [Google Scholar]
[19]. Henderson VW, Popat RA, Effects of endogenous and exogenous oestrogen exposures in midlife and late-life women on episodic memory and executive functionsNeuroscience [Internet] 2011 191Elsevier Inc:129-38. [Google Scholar]
[20]. Nachemson AK, Lundborg G, Myrhage R, Rank F, Nerve regeneration and pharmacological suppression of the scar reaction at the suture site: An experimental study on the effect of oestrogen-progesterone, methylprednisolone-acetate and CIS-hydroxyproline in rat sciatic nerveScand J Plast Reconstr Surg Hand Surg [Internet] 1985 19(3):255-60. [Google Scholar]
[21]. Pascual E, Giner V, Aróstegui A, Conill J, Ruiz MT, Picó A, Higher incidence of carpal tunnel syndrome in oophorectomized womenBr J Rheumatol [Internet] 1991 30(1):60-2.Available from: http://apps.isiknowledge.com/full_record.do?product=UA&search_mode=Refine&qid=5&SID=V1241cja@h8bkCMBnF5&page=26&doc=1290 [Google Scholar]
[22]. Phalen GS, The carpal-tunnel syndrome. Seventeen years’ experience in diagnosis and treatment of six hundred fifty-four handsJ Bone Joint Surg Am [Internet] 1966 48(2):211-28. [Google Scholar]
[23]. Björkqvist SE, Lang AH, Punnonen R, Rauramo L, Carpal Tunnel syndrome in ovariectomized womenActa Obstet Gynecol Scand [Internet] 1977 56(2):127-30. [Google Scholar]
[24]. Kim H, Ku SY, Sung JJ, Kim SH, Choi YM, Kim JG, Association between hormone therapy and nerve conduction study parameters in postmenopausal womenClimacteric [Internet] 2011 14(4):488-91. [Google Scholar]
[25]. Crone C, Krarup C, Neurophysiological approach to disorders of peripheral nerve [Internet]Handbook of Clinical Neurology 2013 1st edElsevier B.V.:81-114.Available from: http://dx [Google Scholar]
[26]. Roberts RO, Cha RH, Knopman DS, Petersen RC, Rocca WA, Postmenopausal oestrogen therapy and Alzheimer disease: overall negative findingsAlzheimer Dis Assoc Disord [Internet] 2006 20(3):141-46. [Google Scholar]
[27]. Krug R, Born J, Rasch B, A 3-day oestrogen treatment improves prefrontal cortex-dependent cognitive function in postmenopausal womenPsychoneuroendocrinology 2006 31(8):965-75. [Google Scholar]
[28]. Leonelli E, Bianchi R, Cavaletti G, Caruso D, Crippa D, Garcia-Segura LM, Progesterone and its derivatives are neuroprotective agents in experimental diabetic neuropathy: A multimodal analysisNeuroscience 2007 144(4):1293-304. [Google Scholar]