With increased demand for apheresis platelets, higher platelet yield and higher donation frequencies were followed to meet the demand. This practice has raised concern on donor platelet depletion. The effects of apheresis donation on donor haematological parameters have been studied more in the west. There remains a conflicting picture on the effect of platelet apheresis on the donor with some studies concluding even repeated platelet apheresis is safe with no significant adverse effects [6] and some reporting significant effect on haematopoiesis [7].
AABB guidelines require an apheresis platelet to have a product count of more than 3x1011 platelets / bag in atleast 90% of the products, whereas the 2007 council of Europe recommends >2 x1011 platelets per haemostatic dose of SDP [8]. Donor can donate platelets at a minimum interval of 48 hours, not more than twice a week and not more than 24 times a year. AABB standards do not require a pre platelet count for single and double apheresis platelet collections. A Pre-donation count is required only if the frequency of donation is within 4 weeks of last donation [9]. The guidelines governing the frequency of plateletpheresis donations are derived from the west, it has to be determined to what extent these guidelines can be applied to Indian population by short term and long term follow-up of these donors.
To analyse the recovery of platelet count to baseline among apheresis platelet donors.
Materials and Methods
A prospective observational study carried out in Department of Transfusion Medicine during the period 2013-2014 with approval from Institutional ethics committee. Fifty apheresis platelet donors were included in the study. The sample size was arrived based on previous years experience on the number of apheresis procedures that were average 48 procedures per year.
Standard operating procedure derived from Director General of Health Services (DGHS) guidelines for aphersis donor selection was followed. Donors who were motivated to donate by apherseis method were requested to fill the Donor questionaire form. Donors were subjected for clinical examination. Haemoglobin was measured by Haemocue. Blood pressure and pulse rate were recorded. Blood sample from donor was sent for platelet count estimation by cell counter to haematology laboratory.
Blood grouping and Rh typing were done. Screening for Transfusion Transmissible Infections on the donors and on the product were done using Chemiluminescence Immunoassay (CLIA).
Considering the pre-donation platelet count of the donor, donors have been categorized into three groups [Table/Fig-1]. The target platelet yield was set at 3×1011 Platelets per collection. Donor and procedure parameters were calculated and results were expressed as Mean±S.D.
Donor groups based on Pre-donation Platelet count.
| S.NO. | DONOR GROUP(S) | DONOR POPULATION | NO. OFDONORS |
|---|
| 1 | I | Pre-donation platelet count 1.5laks/μl to 2.2lacs/μl. | 12 |
| 2 | II | Donors with platelet count >2.2 lacs/μl to 2.75 lacs/μl | 23 |
| 3 | III | Donors with platelet count>2.75 lacs/μl to 3.5 lacs/μl. | 15 |
All procedures were performed with Fresenius Kabi COM.TEC, Germany, Edition 5/06.05, Software version 4.00. It is a single needle intermittent/ continous type of cell seperator. Closed system plateletpheresis kits (S5L) were supplied by manufacturer. Kits were installed according to preset instructions displayed on equipment screen. Kits were primed using Normal saline. Acid Citrate Dextrose (ACD) was the anticoagulant used for the procedure. Donor and procedure parameters were observed. Expected (Estimated) post procedure platelet count and Recruitment Factor (RF) was estimated using the following formula-
Expected (Estimated) post procedure platelet count = Pre-donation platelet count in total blood volume(in 1011) – yield in product bag in (x 1011) expressed as lacs/μl [10].
Recruitment factor = (postdonation cell count + cell yield) / pre-donation cell count/L) [10].
Donors were followed up for platelet count estimation at 30 minutes post-donation, 48 hours, 7th day, 14th day after the procedure. A 3 ml of whole blood was collected from donors in Ethylene Diamine Tetra Acetic Acid (EDTA) vacutainer and sent for platelet count. Platelet count was done using Beckman coulter, Automated LH 780 analyser.
Statistical Analysis
Donor and procedure parameters observed were analysed for statistical significance. Student’s t-test, AVOVA(analysis of variance), One-way and repeated measurement. Pearson’s r-test was used to ascertain linear association between variables. Results were expressed as Mean±S. D and p<0.05 was considered statistically significant.
Results
During the study period, a total of 50 donors were recruited to participate in the study. All donors who were screened for apheresis collection had platelet count above 1.5 lacs/μl. Post-donation platelet count decreased significantly from the baseline in donors across all target yield groups (p<0.0001). The actual post-donation count was higher than expected in group I (p= 0.7). The remaining two groups had a lower post-donation platelet count than expected post-donation platelet count.
Group I with target yield 2 x1011 had the highest recruitment factor 1.02±0.08 [Table/Fig-2]. No significant difference noticed in recruitment factor across all the three groups (p= 0.07) [Table/Fig-3].
Recovery of platelet count in numbers and percentage to baseline.
| Group | PRE COUNT | POST COUNT | Expected Post count | RF | 48 HRS | 7TH DAY | 14TH DAY | PRECOUNT % | POST COUNT % | 48 HRS % | 7TH DAY% | 14TH DAY% |
|---|
| I | 1.99±0.26 | 1.65±0.29 | 1.62±0.18 | 1.02±0.08 | 1.78±0.32 | 2.09±0.34 | 2.2±0.34 | 100±0 | 82.95±7.19 | 89.24±7.74 | 105.04±12.42 | 110.87±12.52 |
| II | 2.58±0.43 | 1.98±0.41 | 2.1±0.37 | 0.958±0.09 | 2.27±0.44 | 2.61±0.40 | 2.70±0.42 | 100±0 | 76.61±9.0 | 87.83±10.3 | 101.77±7.7 | 106.63±7.10 |
| III | 3.12±0.5 | 2.29±0.5 | 2.52±0.61 | 0.955±0.08 | 2.62±0.47 | 2.99±0.46 | 2.18±0.48 | 100±0 | 76±8.6 | 84.2±6.0 | 96.22±5.81 | 102.46±6.0 |
Data presented as Mean±S.D
Note: RF - Recruitment Factor (PRECOUNT-Pre-donation platelet count, POSTCOUNT-post-donation platelet count, RF-Recruitment factor (Recruitment of sequestered platelets from spleen into general circulation is expressed as recruitment factor).
Recruitment factor across the 3 groups.
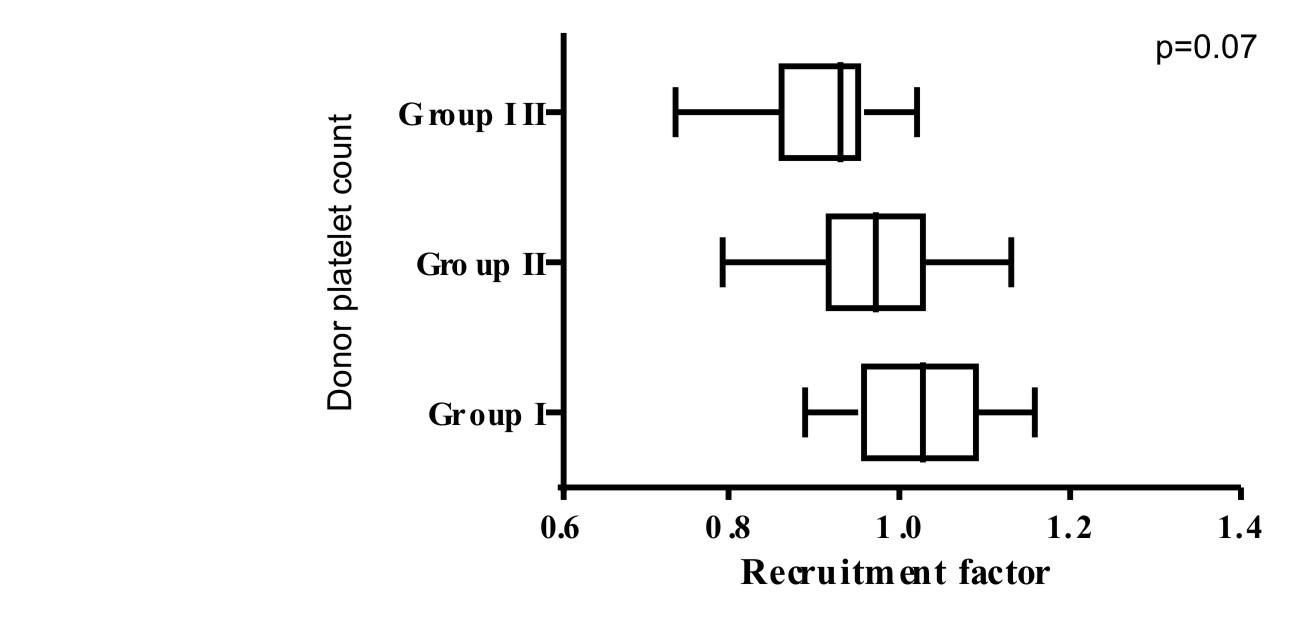
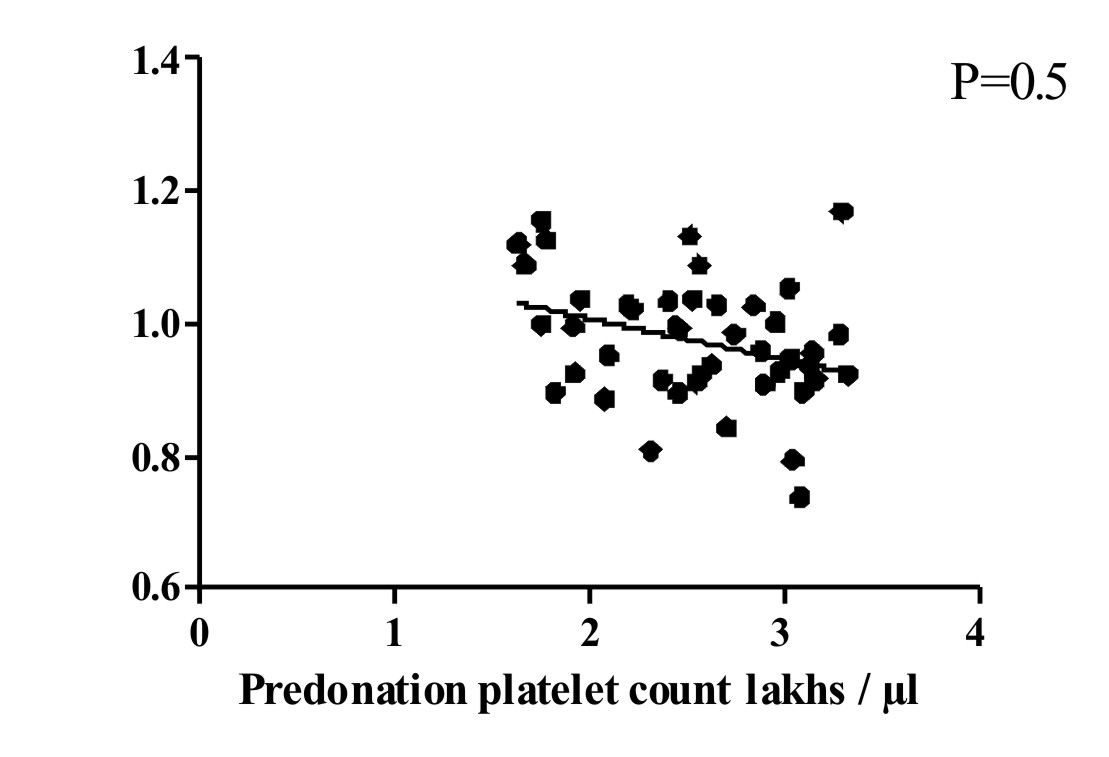
A significant inverse correlation (p=0.04) was observed between Pre-donation count and recruitment factor. As precount increased, the recruitment factor decreased [Table/Fig-4].
Co-relation between precount and recruitment factor.
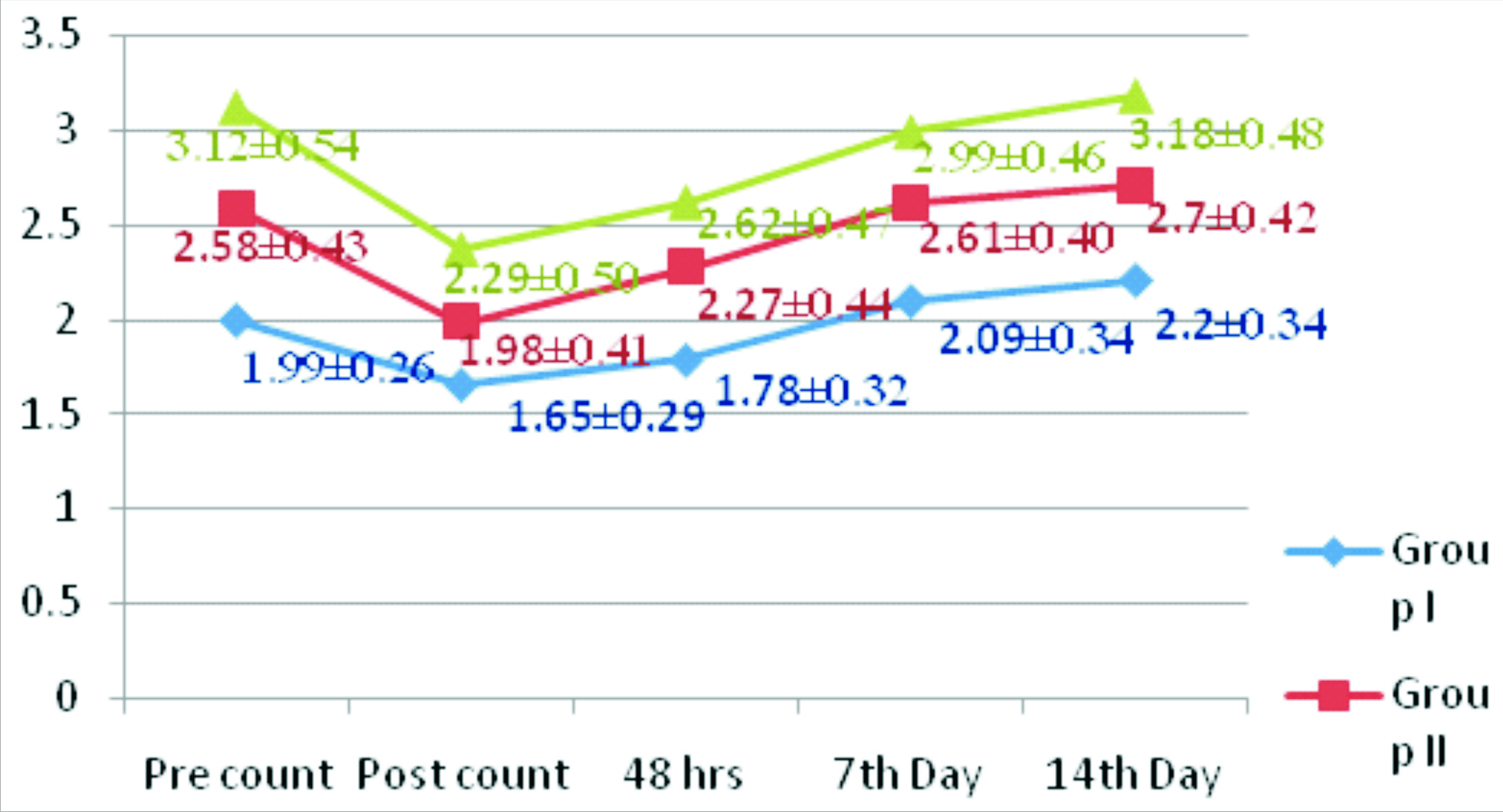
As shown in [Table/Fig-3], the platelet counts measured at various intervals from Pre-donation count to 14th day post-donation count followed a similar trend across all groups except for quantitative difference. Platelet count increased progressively post procedure in all groups during the time period.
On analysing post-donation donor counts across the three groups, donor’s platelet loss ranged from 0.33±0.14 lacs/μl in group I to 0.6±0.02 lacs/μl in group II and 0.72±0.09lacs/μl in group III.
By 48 hours, donor’s platelet count recovered in range from minimum of 84.3% in group III to a maximum of 89.24% in group I with no significant difference in recovery across the three groups.
By day 7, donor’s platelet count showed a deficit of 3.88% in group III to excess of 5% above baseline in group I. Platelet count returned to above the baseline in groups I and II.
By day 14, donor’s platelet count returned to baseline in all three group, with excess of 10% above baseline in group I and 4% above baseline in group II and 2% above baseline in group III (p<0.01) [Table/Fig-4,5].
Platelet count recovery trend in relation to time.
Discussion
The mean pre-donation platelet count among donors in our study was 2.54±0.37. In a similar study done in Southern India, a similar pre-donation platelet count 2.80±0.55 was seen [11]. In a study from North India by SS Das et al., the mean Pre-donation platelet count was lower (2.14±0.53) [4]. In another study from North India the donor population had a pre-donation count of 2.13±0.65 for single dose SDP collection and had 2.82±0.38 in donors selected for double platelet collection [12].
Post-donation, donor’s platelet count dropped significantly in all three groups in the present study. Post-donation platelet count decrease had been demonstrated in many studies. In a study from south India (n=90) with a mean Pre-donation count of 2.80±0.55 for a target yield of 3x1011 platelets donors lost 1.05 lacs/μl of platelets [11]. In this study donors with a mean pre-donation count of 2.54±0.37 lacs/μl, with yield target yield of 3 x1011 experienced a platelet loss of 0.59±0.19 lacs/μl.
In another study (n= 457) a drop in platelet count of 0.77±0.31 was seen in donors with target yield of 3 x1011 [4]. Erwin F. Strasser et al., in 2005 using the same equipment donors with the same target yield experienced a platelet loss of 0.62 lacs/μl (24.5±6.3%) [10].
A platelet count drop by 0.70±0.22 lacs/μl was observed in a study [13]. In this study donors experienced a platelet loss of 22±8.6% which is in agreement with the study compared.
In the present study, donors with low normal platelet count had post-donation platelet count higher than the estimated, a similar observation reported by RL Rogers et al., [14]. In this study, the actual platelet loss was less than expected with group I which had a low baseline platelet count. This group had a recruitment factor >1 which implies some platelet redistribution within the body. The recruitment factor observed is less as the pre-donation platelet count increased.
A study from Germany also observed recruitment factor >1 (1.10±0.14 & 1.20±0.23) in double and triple apheresis procedures respectively [15]. The RF was more than 1 seen in group I as only in this group as the post-donation platelet count was close to lower limit of normal (< 1.5 lacs/μl). A stimulus for recruitment of platelets from spleen would exist only in this group as in other groups the post-donation platelet count was > 1.9 lacs/μl. The depletion of platelet count would not have been sufficiently enough to provide a recruitment signal for spleen for the remaining two groups in our study.
In our study, donors platelet count recovered to baseline by Day 7 in groups I&II. Platelet count recovered to base line by 14th day in our group III. In a study on 352 repeat platelet pheresis donors who underwent atleast four apheresis procedures, it was observed that post-donation donor’s platelet count dropped by 30% below the baseline and returned to base line by four to six days [16].
In another study, the post-pheresis platelet count to return to base line by day 7. The thrombopoetin level increased by day 1 reached and continued to remain elevated even by day 7, colony forming unit –megakaryocytes (CFU-Mk) also showed increase from day 1 reached peak by day 4 and showed a decrease by day 7 [17]. The recovery trend observed in this study was comparable to our study. The increase in serum thrombopoeitin (TPO) and CFU-Mk provides the evidence for the recovery trend seen in platelet count.
A similar study demonstrated 30% decrease in platelet count post pheresis, by day 2 platelet count recovered to 80% of their baseline value, By day 3 and day 4 platelet count increased to 85% of their baseline count and by day 7 platelet counts were somewhat higher than baseline though not significantly [18].
Overall, the present study has been close to baseline or reached baseline by day 7 and a slight increase above the baseline by day 14. The recovery trend in platelet count matches with the increase in thrombopoetin levels mentioned in other studies [17]. With increase in need of apheresis products and advancements in collection technology that is not matched by increase in donor population, the effect of repeated apheresis even within the guidelines may have an effect on the donor parameters, whether the effect of repeated apheresis procedures have an significant clinical relevance or an effect that have no clinical significance have been argued in some studies [17].
Conclusion
Platelet count recovery post-donation showed a similar trend across the three groups of donors. By day 7, donor’s platelet count recovered to baseline in majority of the donors. A similar recovery trend was observed in similar studies. Allowing enough recovery periods for donor platelet count, the minimum interval between two apheresis donations can be seven days till more prospective studies conclude on the frequency and minimum interval between Plateletpheresis donations. The short-term decrease in platelet count following a single apheresis procedure has been found to recover without much clinical significance. The definite answers for the effect of frequent apheresis on donors haematopoiesis has to come from a long–term registry of repeat apheresis donors.
Data presented as Mean±S.D
Note: RF - Recruitment Factor (PRECOUNT-Pre-donation platelet count, POSTCOUNT-post-donation platelet count, RF-Recruitment factor (Recruitment of sequestered platelets from spleen into general circulation is expressed as recruitment factor).