Erectile dysfunction is a well-recognized complication in diabetes mellitus and it is common in older men. Also, decrease libido and orgasmic dysfunction are commonly associated with erectile dysfunction in patients with type 2 Diabetes Mellitus (DM) [1,2]. Sex drive and desire are regulated by sex hormones and affected by psychological factors and metabolic diseases like DM [3].
Type 2 DM is an endocrine disorder accounting for 90% of diabetes cases, characterized by hyperglycaemia, polyuria, polydepsia, polyphagia and weight loss due to impaired insulin action and/or secretion [4]. The role of insulin is essential for glucose homeostasis via induction of peripheral tissue glucose uptake, inhibiting hepatic glucose output and regulating lipid metabolism in cooperation with other hormones like catecholamine, cortisol, growth hormone and glucagon [5].
Recently, higher endogenous testosterone secretion has been correlated with beneficial effect on cardiometabolic profile through induction of High Density Lipoproteins (HDL) and reduction in serum levels of cholesterol and triglyceride, whereas exogenous testosterone leads to harmful effects causing dyslipidemia and various hepatic dysfunctions [6].
Likewise, low testosterone is associated with the pathogenesis of type 2 DM. Recent studies demonstrated that men with type 2 DM have low testosterone levels that cause low sex drive and erectile dysfunction [7,8].
Moreover, low testosterone serum levels are more linked with type 2 DM coupled with obesity causing sexual dysfunction, physical fatigue and mood changes [9]. Furthermore, other factors like increasing age, impaired penile blood flow and peripheral neuropathy with low testosterone are often responsible for type 2 DM-induced erectile dysfunction [10]. Indeed, patients with diabetic-induced erectile dysfunction with low testosterone respond weakly to sildenafil and respond better to the testosterone replacement therapy that also causes improvement in insulin sensitivity, glucose and lipid homeostasis [11].
Men with type 2 DM are found to have hypogonadism and high Body Mass Index (BMI), since there is an inverse corelation between free or total testosterone with high BMI [12] in addition; Sex Hormone Binding Globulin (SHBG) is low in patient with type 2 DM with and without obesity [13].
Many longitudinal studies demonstrated that men with low testosterone levels are at higher risk for development of type 2 DM due to induction of insulin resistance, since free testosterone levels are inversely corelated with insulin resistance [14,15].
Metformin is an insulin sensitizing agent that increases insulin sensitivity and reduced androgen serum levels through inhibition of ovarian gluconeogenesis in women with polycystic ovary syndrome [16].
Furthermore, metformin therapy leads to significant reduction in testosterone serum levels in diabetic and non-diabetic patients [17]; while, sulfonylurea (glimepiride) restore testosterone serum levels in type 2 DM patients [18].
Thus, the aim of the present study was to evaluate the erectile dysfunction and sex drive in relation to testosterone serum levels in type 2 DM and also scrutinize the potential role of diabetic pharmacotherapy {metformin versus sulfonylurea (glibenclamide)} upon these changes.
Materials and Methods
This case control study was carried out in the Department of Clinical Pharmacology and Therapeutic, College of Medicine, Al-Mustansiriyia University in collaboration with the Iraqi Endocrinology Center from January to February 2016 in Baghdad-Iraq. This study was permitted and approved by the Ethics Committee, Al-Mustansiriyia Medical College under ethical clearance number DD208 PT 2/1/2016. The study procedures were done in respect to the Declaration of Helsinki [19].
A total number of 64 patients with well diagnosed type 2 DM (the range of disease duration was 7 years) associated with erectile dysfunction on either metformin or glibenclamide therapy were recruited from the Iraqi Endocrinology Center involved in this study. They were divided into Group (A):34 patients treated with metformin 500mg tid (metformin hydrochloride tablet USA, corona CA 92880 USA), Group (B): 30 patients treated with sulfonylurea (Glibenclamide) 5mg/day (Daonil tablet, AVENTIS PHARMA LIMITED, VERNA, Goa-403722) and Group (C): 27 healthy normal non-diabetic men subjects that are regarded as control, all controlled subjects were recruited from healthy medical staff at hospital. The duration of treatment for those patients ranged from 4-9 years. All patients were followed-up for one month duration during the study protocol for final findings regarding sex drive and full medical history like the response to sildenafil, drug allergy, pelvic trauma and psychological disorders.
Inclusion criteria: Male patients with type 2 DM without complications, with age range of 41-49 years and on treatment with either metformin or glibenclamide that are associated with erectile dysfunction.
Exclusion criteria: Testosterone replacement therapy, malignant diseases, psychological disorders, neurological disorders, renal dysfunction, hepatic dysfunction, connective tissue diseases, congenital hypogonadism, thyroid dysfunctions, cardiovascular complications and patients on drugs that induce sexual dysfunction like diuretics and β-blockers.
Anthropometric measurements
Height and body weight were estimated by specific stadiometer and calibrated digital balance respectively, then BMI was calculated as BMI=weight (kg)/height (m2), also Waist-Hip Ratio (WHR) was determined [20]. Furthermore, blood pressure measurement was done by mercury sphygmomanometer.
Biochemical analysis
After an overnight fasting 10 ml of blood was drawn from an antecubital vein under aseptic condition, then after centrifugation at 3000rpm the serum was stored at -20°C for further analysis. Fasting Blood Glucose (FBG) mg/dL was determined by specific method (fasting capillary blood glucose) [21], while lipid profile was determined by Enzyme-Linked Immunosorbent Assay (ELISA) kit method according to the kit instruction, and then Very Low Density Lipoproteins (VLDL) and Low Density Lipoproteins (LDL) were estimated by Friedwald formula [22]. Besides, atherogenic index was evaluated depending on total triglyceride and high density lipoprotein measured in mmol/L AI=log (Total Triglycerides/HDL) [23].
Hormonal assay
Total testosterone (TT) in nmol/L was measured by specific ELISA kit method (ADI-901-065, ENZO immunoassay) [24].
SHBG in nmol/L was measured by specific ELISA kit method (Human SHBG, ELSA Kit; MGC126834).
Free androgenic index was estimated as 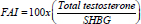 , (normal value 30-150), additionally, free and bioavailable testosterone were estimated according to Ozata et al., and Vermeulen et al., methods [25,26].
, (normal value 30-150), additionally, free and bioavailable testosterone were estimated according to Ozata et al., and Vermeulen et al., methods [25,26].
Assessment of erectile dysfunction
This was done by using the Sexual Health Inventory for Men (SHIM) which is a patient self administrated questionnaire for assessment of male sexual dysfunction, scoring from 1-21 scores, where; 1-7 score= severe erectile dysfunction, 8-11=moderate erectile dysfunction, 12-16= moderate erectile dysfunction, 17-21=mild erectile dysfunction and >21= normal sexual function with 98% sensitivity and 88% specificity [27].
Statistical Analysis
Data of the present study were analysed by means of SPSS version 19; the data were expressed as mean±SD, number and percentage. The unpaired student t-test was used to compare the significance of differences between the diabetic patients and non-diabetic healthy control, whereas one-way ANOVA test was used to detect the significance of differences between treated groups as compared to the control. The p-value <0.05 was considered to be significant, also correlation coefficient was used for evaluating the correlation of SHIM with testosterone variables.
Results
A total number of 70 patients with type 2 DM were screened for enrollment in this study, 6 patients were excluded, so 64 men patients were enrolled and randomized into two treated groups, metformin and sulfonylurea (Glibenclamide) groups compared to 27 normal healthy men regarded as control. Furthermore, all patients and control subjects completed the follow-up [Table/Fig-1].
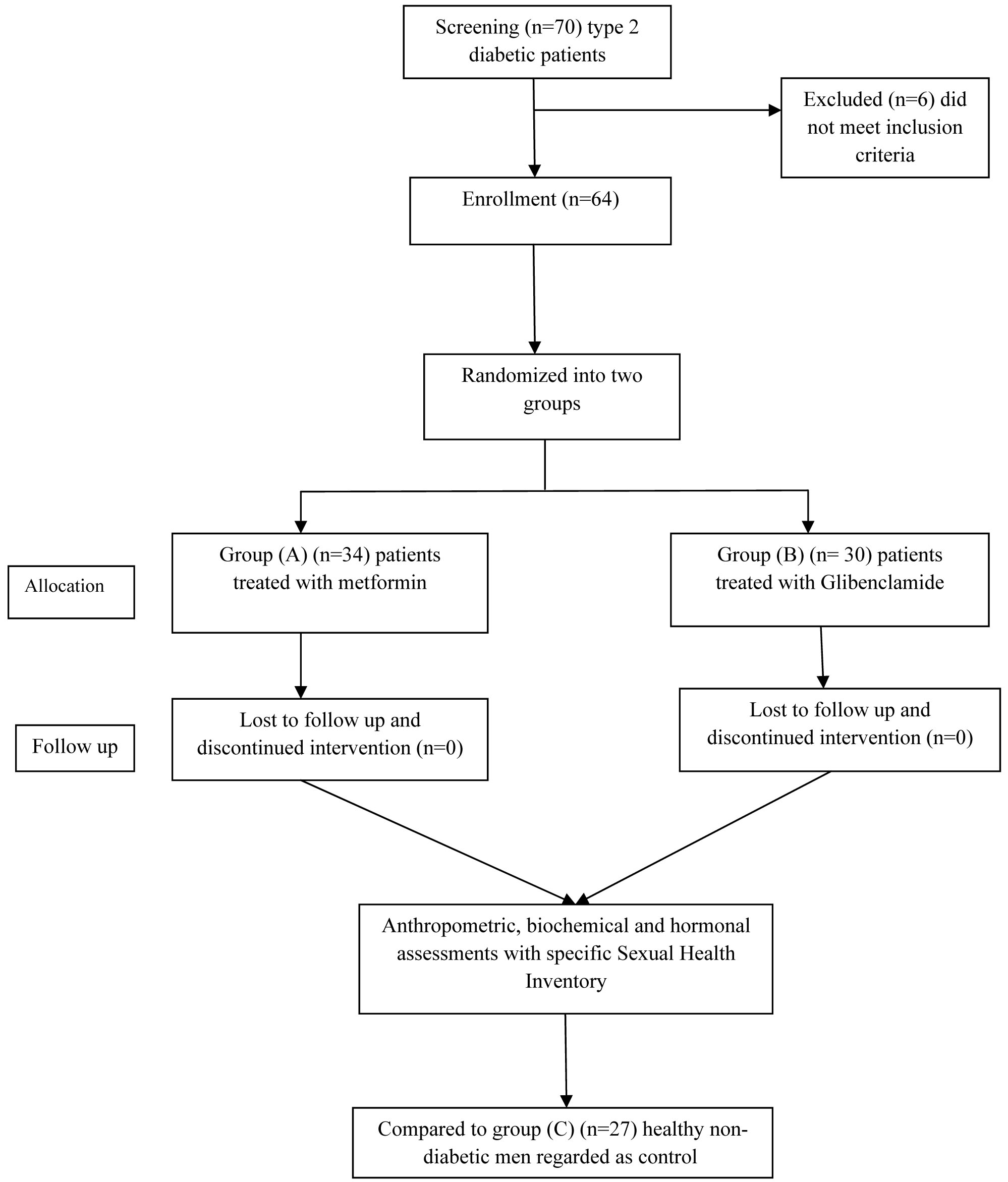
The clinical data of enrolled patients are summarized in [Table/Fig-2]. Age of the patients was ranged from 41-49 years with 6.71±1.27 years as duration of type 2 DM. The results of the present study also revealed that 67.18% and 68.75% of patients had dyslipidemia and hypertension respectively; also 60.93% patients were smokers. Moreover, 75% of men with type 2 DM had erectile dysfunctions, 31.25% of them respond to sildenafil whereas 43.75% of them did not respond to sildenafil therapy as per the medical history.
Changes in the Anthropometric profile
There was no significant difference in age of patients between the three groups. BMI was higher in sulfonylurea treated group compared with metformin treated group, but BMI in metformin treated patients was higher compared to the control whereas; intergroup differences were insignificant p=0.23. Waist circumference was low in control group compared to metformin and glibenclamide treated patients whereas; intergroup differences were insignificant p=0.28. WHR was high in sulfonylurea treated group which significantly differed from metformin treated and control groups p<0.0001, whereas intergroup differences were insignificant p=0.62. Systolic and diastolic (BP) were higher in diabetic patients compared to the control group p=0.01 and p=0.008 respectively, but BP value was low in metformin treated patients compared to glibenclamide treated patients p < 0.0001 [Table/Fig-3].
Demographic characteristics of diabetic patients.
| The characteristics | Total number of diabetic patients | Group A(Metformin) | Group B(Glibenclamide) |
|---|
| NumberAge (years)Duration of type 2 DM(years)Anti-diabetic medicationsDyslipidemiaHypertensionSmokingOther pharmacotherapyAnti-plateletsACEICalcium channel blockersMultivitaminsErectile dysfunctionDuration (months)Respond to sildenafilNot respond to sildenafil | 6441-496.71±1.2764(100.00)43(67.18)44(68.75)39(60.93)22(34.37)42(65.62)22(34.37)29(45.31)48(75)6.54±2.6720(31.25)28(43.75) | 3445.75±4.036.71±1.2734(53.13)16(40.5)20(52.94)19(55.88)12(35.29)24(70.58)9(26.47)10(29.41)30(88.23)7.34±1.656(17.64)20(85.82) | 3043.61±5.885.22±2.2730(46.87)27(90.0)24(80.66)20(66.66)-10(33.33)18(60.0)13(43.33)19(63.33)18(60.0)5.88±2.1114(46.66)10(33.33) |
Results are expressed as Mean±SD, n (%), ACEI: angiotensin converting enzyme inhibitor
Effects of metformin and glibenclamide on anthropometric and blood pressure profile on men patients with type 2 DM compared to normal healthy subjects.
| Variables | Control(n=27) | Metformin(n=34) | Glibenclamide(n=30) | p-value (95% CI lower- upper limits) | p-value |
|---|
| A | B | C |
|---|
| Age (years)BMI(kg/m2)WC(cm)WHRSBP(mmHg)DBP(mmHg) | 44.64±4.9524.53±3.6172.69±9.330.87±0.04121.66±5.6371.33±6.89 | 45.75±4.0327.63±4.6279.83±10.590.92±0.03141.29±11.8192.69±7.33 | 43.61±5.8831.42±6.5585.73±11.720.99±0.03154.74±12.2794.11±9.73 | 0.35(-3.47-1.25)0.004(-5.20-0.99)*0.009(-12.42-1.85)*< 0.0001(-0.06-0.03)*< 0.0001(-24.08-15.17)*< 0.0001(-24.94-17.77)* | 0.47(-1.84-3.90)< 0.0001(-9.67-4.10)*< 0.0001(-18.63-7.44)*< 0.0001(-0.13-0.10)*< 0.0001(-37.67-28.48)*< 0.0001(-26.93-18.62)* | 0.09(-0.42-4.70)0.01(-6.66-0.91)0.03(-11.51-0.28)< 0.0001(-0.08-0.05)*< 0.0001(-19.49-7.40)*0.516(-5.78-2.94) | 0.940.230.280.620.010.008* |
Results are expressed as mean±SD, p-value calculated according to unpaired t-test and ANOVA test, p<0.05,*p< 0.01, A (metformin versus control), B (Glibenclamide versus control), C (metformin versus glibenclamide), BMI: body mass index; WC: waist circumference; WHR: waist hip ratio; SBP: systolic blood pressure; DBP:diastolic blood pressure.
Changes in lipid profile and blood glucose
Lipid profile was higher in diabetic patients compared to the control p < 0.0001, but it was low in metformin treated patients compared to sulfonylurea treated patients significantly p=0.01. Fasting blood glucose and glycated haemoglobin HbA1c in metformin treated patients were low compared to sulfonylurea treated patients, while in sulfonylurea treated patients both fasting blood glucose and HbA1c were higher compared to the control [Table/Fig-4].
Effects of metformin and glibenclamide on glucose levels and lipid profile, on men patients with type 2 DM compared to normal healthy subjects.
| Variables | Control(n=27) | Metformin(n=34) | Glibenclamide(n=30) | p-value (95% CI lower- upper limits) | p-value |
|---|
| A | B | C |
|---|
| TC(mg/dL)TG(mg/dL)HDL(mg/dL)LDL(mg/dL)VLDL(mg/dL)AIFBG(mg/dL)HbA1c (%) | 133.63±11.83132.80±13.7362.44±7.7144.62±9.8426.56±5.560.032±0.0189.31±7.724.23±1.11 | 144.53±22.73166.71±13.9449.84±9.6561.34±9.2233.34±6.390.164±0.02132.52±12.526.73±2.6 | 200.73±12.88189.61±22.8240.99±5.94121.81±13.5537.92±7.330.305±0.05146.71±16.88.22±3.52 | 0.015(-19.66-2.13)< 0.0001(-40.91-26.90)*< 0.0001(8.25-16.94)*< 0.0001(-21.58-11.85)*< 0.0001(-9.77-3.78)*< 0.0001(-0.13-0.12)*< 0.0001(-48.28-38.13)*< 0.0001(-3.45-1.54)* | < 0.0001(-73.32-60.87)*< 0.0001(-65.98-47.63)*< 0.0001(17.87-25.02)*< 0.0001(-83.03-71.34)*< 0.0001(-14.58-8.13)*< 0.0001(-0.29-0.25)*< 0.0001(-63.68-51.11)*< 0.0001-5.23--2.74)* | < 0.0001(-65.32-47.07)*< 0.0001(-32.56-13.23)*< 0.0001(4.88-12.81)*< 0.0001(-66.36-54.57)*0.01(-8.04-1.11)< 0.0001(-0.16-0.12)*0.0004(-21.69-6.68)*0.06(-3.05-0.07) | 0.002*0.010.030.0006*0.160.0005*0.003*0.58 |
Results are expressed as mean±SD, p-value calculated according to unpaired t-test and ANOVA test, p<0.05,*p< 0.01, A (metformin versus control), B (Glibenclamide versus control), C (metformin versus Glibenclamide, TC: total cholesterol; TG: triglyceride; HDL: high density lipoprotein; LDL: low density lipoprotein; VLDL: very low density lipoprotein; AI: atherogenic index; FBG: fasting blood glucose; HbA1c: haemoglobin A1c; Total T: total testosterone.
Changes in serum testosterone levels and Sexual Health Inventory for Men (SHIM)
Total testosterone serum levels were high in glibenclamide treated patients compared to metformin treated patients p < 0.0001 and low compared to the control p < 0.0001. SHBG levels were significantly higher in glibenclamide treated patients compared to metformin treated patients. FAI and FT was significantly higher in glibenclamide treated patients compared to metformin treated patients. Bioavailable testosterone (BT) was low in metformin treated patients compared to the control and glibenclamide treated patients, intergroup differences was insignificant p=0.07. SHIM was low in metformin treated patients compared to the control and glibenclamide treated patients p < 0.0001, intergroup differences was significant p=0.001[Table/Fig-5].
Effects of metformin and glibenclamide on testosterone serum levels and Sexual Health Inventory in men patients with type 2 DM compared to normal healthy subjects.
| Variables | Control(n=27) | Metformin(n=34) | Glibenclamide(n=30) | p-value (95% CI lower- upper limits) | p-value |
|---|
| A | B | C |
|---|
| Total T(nmol/L)SHBG(nmol/L)FAIFT (nmol/L)BT(nmol/L)SHIM | 19.22±4.5255.89±12.9334.38±6.510.289±0.126.78±2.4424.62±2.55 | 6.99±3.9740.62±10.3817.20±4.740.117±0.112.75±1.1210.61±3.22 | 12.23±4.7552.81±9.5123.15±6.490.18±0.094.22±2.7417.73±1.91 | < 0.0001(10.04-14.41)*< 0.0001(9.20-21.33)*< 0.0001(14.20-20.15)*< 0.0001(0.11-0.23)*< 0.0001(3.005-5.05)*< 0.0001(12.56-15.45)* | < 0.0001(4.64-9.33)*0.30(-2.83-8.99)< 0.0001(7.93-14.52)*0.0002(0.05-0.16)*0.0002(1.25-3.86)*< 0.0001(5.71-8.06)* | < 0.0001(-7.44-3.03)*< 0.0001(-17.16-7.21)*0.0001(-8.83-3.06)*0.014(-0.11-0.01)0.0093(-2.55-0.38)*< 0.0001(-8.42-5.81)* | 0.0190.250.020.0005*0.070.001* |
Results are expressed as mean±SD, p-value calculated according to unpaired t-test and ANOVA test, p<0.05,*p< 0.01, A (metformin versus control), B (Glibenclamide versus control), C (metformin versus Glibenclamide), Total T: total testosterone; SHBG: sex hormone binding globulin; FAI: free androgenic index; FT: free testosterone; BT: bioavailable testosterone; SHIM: Sexual Health Inventory for Men.
Sexual health inventory for men is positively correlated with TT, FAI, FT and BT but negatively correlated with SHBG in diabetic patients and healthy men, these correlations were significant except for BT. Also, FT significantly correlated only in control and glibenclamide treated groups but not in metformin treated group, thus; SHIM was testosterone dependent [Table/Fig-6].
Correlations of sexual health inventory for men with serum testosterone levels.
| Variables | Control (n=27) | Metformin (n=34) | glibenclamide (n=30) |
|---|
| r | p | r | p | r | p |
|---|
| Total T(nmol/L)SHBG(nmol/L)FAIBT(nmol/L)FT (nmol/L) | 0.99-0.3790.9940.1010.556 | <0.000010.051<0.000010.6160.0025 | 0.999-0.9990.9990.0360.280 | <0.00001<0.00001<0.000010.830.108 | 0.991-0.4210.99980.0990.399 | <0.000010.02*<0.000010.600.02* |
*p<0.05, p<0.01Total T: total testosterone; SHBG: sex hormone binding globulin; FAI: free androgenic index; FT: free testosterone; BT: bioavailable testosterone; SHIM: Sexual Health Inventory for Men.
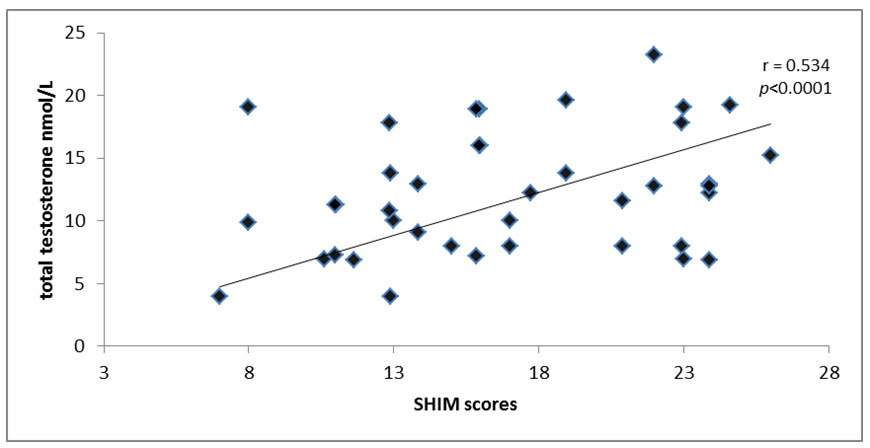
Total testosterone serum levels in diabetic patients and control subjects is significantly correlated with SHIM p < 0.0001, r=0.534 [Table/Fig-7].
Correlation between total testosterone and sexual health inventory for men.
Discussion
The present study demonstrated that men patients with type 2 DM are associated with low testosterone serum levels compared to normal healthy men, this finding is compatible with Cheung et al., findings that indicated a significant proportion of men with type 2 DM had a low testosterone serum levels [28]. Low testosterone serum levels is linked with the occurrence of type 2 DM, since testosterone lead to increments in the muscle mass and decrement in the fat mass that causes a significant reduction in insulin resistance and prevention of type 2 DM. Additionally, testosterone inhibits lipoprotein lipase activity and augment triglyceride uptake that prevents insulin resistance [29,30].
In present study, all enrolled patients were of the middle age group. This was done to exclude the effect of aging on testosterone serum levels since; aging leads to reduction in the androgen serum levels due to defects in testicular-pituitary-hypothalamic axis [31].
Indeed, most of our patients were overweight and obese. Zhao et al., study revealed that low testosterone levels were directly linked to obesity and high BMI since adipose tissue induced-oxidative stress and high levels of adipokine like leptin inhibit-testosterone secretions via direct inhibition of testicular Leydig cell function. In addition peripheral conversion of testosterone to estrogen is augmented causing negative feedback inhibition on luteinizing hormone production and then inhibition of testicular androgen production [32]. This leads to sexual dysfunction and low sex drive as demonstrated in the present study.
Regarding BP changes, about 44% of enrolled diabetic patients with low testosterone levels were hypertensive, which may be due to coexisting diseases or to the low androgen serum levels as supported by Zheng et al., a study that pointed out to the association of low testosterone serum levels with incidence of atherosclerosis and hypertension in middle age men [33]. Moreover, testosterone therapy is more effective in reducing BP than exercise and diet alone in metabolic syndrome [34].
Additionally, in the present study about 43% of type 2 DM patients with subnormal testosterone levels presented with dyslipidemia, this association was supported by many studies that disclosed an association between low testosterone and risk of dyslipidemia. A study indicated that androgen deprivation therapy in treatment of prostatic cancer leads to hyperlipidemia and increases the risk of cardiovascular complications [35], whereas another study showed that androgen deprivation therapy causes an early elevation in total cholesterol and TG levels [36].
Furthermore, the present study showed that fasting blood glucose and HbA1c were higher in diabetic men patients with low testosterone serum levels compared to the control men, since testosterone inhibits gluconeogenic and glycogenolytic pathways, decreases corticosterone effects on glucose metabolism, improves skeletal muscle glucose uptake and inhibits hepatic insulin resistance [37]. Thus, reduction in testosterone levels in type 2 DM may elevate fasting blood glucose and subsequently HbA1c as observed in our study.
Certainly, 39% of our patients were chronic active smokers with low testosterone serum levels. This finding was incompatible with Lotti et al., who found an elevation in testosterone levels in chronic smokers which may be due to reduction in testosterone peripheral sensitivity, luteinizing hormone stimulation, stimulation of testosterone production and inhibition of peripheral conversion of testosterone to estrogen [38]. The possible explanation for low testosterone in the present study compared to Lotti et al., can be that, most of our patients were diabetic with higher BMI and randomly selected from general population while Lotti et al., chose infertile non-diabetic patients from a single infertility center .
Regarding the effect of diabetic pharmacotherapy on testosterone serum levels, metformin significantly reduces TT; through inhibition of Cytochrome P450-C17a which is a key enzyme in TT synthesis and reduction of LH hormone secretion [39]. Also, metformin therapy in type 2 DM leads to significant reduction in total TT, FT, BT and FAI through modulation of leptin secretion [40]. In normal men, short-term administration of metformin leads to significant reduction in TT, FT and increases in SHBG [41]. All of these findings are compatible with the results of the present study.
On the other hand, TT, FT, BT levels and FAI were significantly higher in patients treated with sulfonylurea compared to metformin treated patients, that corresponding with a study by Wong et al. They found that treatment with sulfonylurea in type 2 DM led to significant elevation in TT and FT due to inhibition of pro-11b-hydroxysteroid dehydrogenase type 1 leading to reduction in glucocorticoid biosynthesis and stimulation of testosterone synthesis since; thus glucocorticoid reduces testosterone levels [42]. Moreover, it is well-known that sulfonylureas stimulate insulin secretion which plays an important role in the regulation of testicular function and hypothalamic-pituitary-testicular axis, thus insulin improves testosterone levels and testosterone secretion index in type 2 DM [43].
In the present study, sulfonylurea showed an insignificant effect on SHBG levels compared to metformin, this finding is supported by previous studies that demonstrated non-significant effect of sulfonylurea on SHBG in type 2 DM compared to the baseline data, since SHBG reduces testosterone bioavailability and because insulin inhibits hepatic SHBG production so BT is augmented [44,45].
Additionally, scores of the SHIM were significantly higher in sulfonylurea treated patients compared to metformin treated patients; this indicated that sex drive was superior in sulfonylurea treated patients compared to metformin treated patients, since testosterone improves sex drive in hypogonadal men [46].
Furthermore, insulin improves testosterone serum levels, causing increasing sex drive and reduction of erectile dysfunction as well. Tight glycemic control by sulfonylurea plus 5-phosphodiestrase inhibitor improved sexual function more than 5-phosphodiestrase inhibitor alone in experimental animals due to the synergistic effects on penile nitric oxide [47]. In contrast, Rey-Valzacchi et al., reported that addition of metformin to sildenafil improves diabetic-induced erectile dysfunction [48].
Consequently, low sex drive and erectile dysfunction in type 2 DM are multifactorial with and without low testosterone levels [7], also; metformin improves erectile function in insulin resistance and diabetic-induced erectile dysfunction inspite of low testosterone levels [49], but the reverse is documented in the present study.
SHIM is positively correlated with TT, FT and negatively correlated with SHBG in both metformin and sulfonylurea treated patients, without significant correlation with BT [50].
Limitation
Firstly, patients could not be followed-up for a longer time period for evaluating the effect of metformin or sulfonylurea on testosterone levels. Secondly, the sample size of diabetic patients was small. Thirdly, serum insulin and insulin resistance were not evaluated and correlated with TT, since they are well and extensively studied in previous researches.
But, regardless of these limitations, to our knowledge this study may be the first one that implicated metformin in low sex drive and erectile dysfunction in type 2 DM.
Conclusion
Metformin in type 2 DM leads to significant reduction in testosterone levels, sex drive and induction of low testosterone-induced erectile dysfunction, whereas; sulfonylurea in type 2 DM leads to significant rise in testosterone levels, sex drive and erectile function.