Delayed secondary damage is one of the mechanisms associated with poor prognosis in patients with Traumatic Brain Injury (TBI) and usually occurs due to biochemical and structural changes in the brain [1]. Oxidative stress, as a trend, is associated with delayed secondary damage in TBI patients and is known to play a substantial role in the development and progress of diseases in the Central Nervous System (CNS), especially TBI [2,3]. Nowadays, numerous studies are being conducted to investigate the potential of oxidative stress biomarkers in predicting prognosis and for a better management of TBI patients [4]. Human brain, compared with other organs, releases smaller amounts of endogenous antioxidants when faced with oxidative stress and it is therefore more susceptible to the effects of oxidative stress [5]. Uric Acid (UA) is an antioxidant with hydrophilic and androgenic properties and its serum levels are modified by both drug and diet [6–8]. UA is responsible for more than 50% of free radical scavenging activities [9] by removing superoxide and singlet oxygen and protecting oxidation of vitamin C by iron chelation [10–11]. Peroxynitrite and hydroxyl radicals induce membrane lipid peroxidation and initiate the autocatalytic process of cerebral ischaemic injury [12,13]. In-vitro studies have shown that UA inhibits processes related to hydroxyl radical and peroxynitrite induced damages [14,15]. Epidemiological studies have shown that high UA levels are associated with better prognosis in stroke patients and in acute stroke patients under thrombolytic therapy [9]. Studies have also reported the neuroprotective effects of UA in animal models of focal ischaemic injury [16,17]. So far, different studies have been conducted on the relationship between UA levels and the prognosis of patients with TBI. The aim of our study was to investigate the relationship between serum UA levels and prognosis of patients with TBI during hospitalization and six months after discharge.
Materials and Methods
Study Design: All patients who attended our emergency department during July 2014 and December 2015 were entered into the study consecutively and among 890 evaluated candidates and based on inclusion criteria 725 TBI patients were finally selected for further investigation. This prospective cohort study was conducted on 725 patients with TBI who were admitted to the emergency department of Imam Khomeini Hospital in Ilam City, Iran. The inclusion criteria were as follow: Severe TBI based on an admission Glasgow Coma Score (GCS) <8 with positive finding on head Computed Tomography (CT), sustained a non-penetrating trauma to the head within the previous 4 hours; age between 18 and 65 years; availability for follow-up appointments. This medical center, situated in the west of Iran, is a referral center for patients with trauma and covers a population of about 7,00,000. The present study was approved by the ethics committee of the Ilam University of Medical Sciences, Ilam, Iran. All study objectives were explained to the patients and if necessary, to the first-degree relatives of those patients with low level of consciousness. By oral interview and a written consent form, patients’ satisfaction was obtained from all patients participating in the study. Clinical and demographic data of patients, including sex, age, body mass index, history of chronic diseases and data related to TBI, such as mechanism of injury, type of intracranial injury and GCS score, were obtained for all patients. GCS assessment was performed for all patients via a previous protocol [18]. Upon arrival of patients to the emergency department, primary care rehabilitation and treatment were started as required by the existing guidelines [19], including checking of airway and, if necessary, intubation, controlling the blood pressure and pulse rate, fluid therapy, determining the GCS score and measuring laboratory parameters if needed for the insertion of extra-ventricular drain, central venous catheter and arterial catheters. TBI was confirmed by the CT scan findings according to the Marshall protocol [20]. CT scan was repeated, if necessary in patients with altered consciousness, focal neurological symptoms and signs, seizure and status epilepticus. The exclusion criteria included patients with multiple trauma, pregnancy, use of oral contraceptives, hormone therapy or anticoagulant therapy, presence of coagulopathy or bleeding disorders, endocrine or metabolism disorders, alcohol or drug abuse, systemic diseases of the lung, heart, liver or kidney, diabetes or neurodegenerative diseases, age below 20 years and voluntary withdrawal at any time until the end of the study.
Evaluation of Outcomes: For outcome evaluation of TBI patients, the GOS score was used, according to which the prognosis of patients was typically divided into five groups: 5=good recovery, 4=moderate disability, 3=severe disability, 2=persistent vegetative state and 1=death. In this study; however, the patients were divided into two categories: death group with a GOS score of 1 and survival group with a GOS score of 2 to 5 during hospitalization and 6 months after discharge from the hospital [21].
Laboratory Analysis: Blood sampling was done on the first day after TBI for the cases and for all subjects when they were enrolled in the study. Sera were isolated from the peripheral blood samples taken from each subject at the set time points. Blood samples were centrifuged at 3000 rpm for 10 minutes. Before biochemical measurements; each serum sample was frozen at −80°C.
Statistical Analysis
All statistical analyses were performed using SPSS software version 19) (SPSS Inc., Chicago, USA). Initially, the normality of data was measured using the Kolmogorov–Smirnov analysis test. Data are presented as mean±SD for quantitative variables and as frequencies for qualitative variables. The Student’s t-test and chi-square test were used for data analysis. Correlations between serum UA levels and the severity of brain injury were assessed by Marshall’s classification. Also, correlations between UA levels and patient outcome were evaluated according to the patients’ GOS score. In addition, the Receiver-Operator Characteristic (ROC) curves associated with different serum UA levels were constructed for both the time points, either in-hospital or 6 months after discharge. All p-values less than 0.05 were considered statistically significant for all variables.
Results
[Table/Fig-1] shows the clinical and demographic characteristics of participants. In total, data of 725 participants were analyzed. Of these, 311 participants (42.89%) were men and 414 (57.10%) were women. The mean and SD of age and BMI for all participants were 54.69±12.37 years and 24.35±3.54kg/m2, respectively. Blood pressure of patients on arrival at the emergency department was 105.43±15.34mmHg. The most frequent cause of trauma was motor vehicle accidents (241 cases; 33.24%), followed by falling down (164 cases, 22.62%). The other causes of TBI included, motorcycle accidents (84 cases; 11.58%), gunshot injuries (52 cases; 7.17%), assault (120 cases; 16.55%), and others (64 cases; 8.82%). Based on the severity of TBI and according to the Marshall’s scoring system, the frequency of diffuse type I injury was 303 cases (41.79%); diffuse type II, 102 cases (14.06%); diffuse type III, 152 cases (20.96%); and diffuse type IV, 52 cases (7.17%). Moreover, evacuated focal mass lesion type V was observed in 72 cases (9.92%) and focal mass lesion type VI in 44 cases (6.06%). The mean±SD of GCS score of patients was 4.65±1.76. The mean±SD of days of hospitalization and ICU stay was 12.11±3.45 days and 4.54±2.76 days, respectively. Analyzing the outcome, our results showed that 247 cases (34.06%) died during hospitalization and 478 cases (65.93%), who survived were discharged from hospital. At the six-month follow-up of patients after hospital discharge, 128 patients (17.65%) died and 350 (48.27%) survived.
Clinical characteristic of patients.
| Varaibles | TBI, n=725 |
|---|
| Sex, n (%) |
| Male | 311(42.89) |
| Female | 414(57.10) |
| Age (y) | 54.69±12.37 |
| BMI (kg/m2) | 24.35±3.54 |
| BP (mmHg) | 105.43±15.34 |
| Injury mechanism |
| Motor Vehicle Accident | 241(33.24) |
| Motor Cycle Accident | 84(11.58) |
| Gun Shot Wound | 52(7.17) |
| Fall | 164(22.62) |
| Assault | 120(16.55) |
| Other | 64(8.82) |
| GCS, median (range) | 4.65±1.76 |
| Marshall score, n (%) |
| Diffuse I | 303 (41.79) |
| Diffuse II | 102 (14.06) |
| Diffuse III | 152 (20.96) |
| Diffuse IV | 52 (7.17) |
| Evacuated focal mass lesion V | 72 (9.93) |
| Focal mass lesion VI | 44 (6.06) |
| Days in hospitalization | 12.11±3.45 |
| Days in intensive care units | 4.54±2.76 |
| Outcome in-hospital, n (%) |
| Deceased (GOS 1) | 247 (34.06) |
| Alive (GOS 2-5) | 478 (65.93) |
| Outcome in 6 months, n (%) |
| Deceased (GOS 1) | 128 (17.65) |
| Alive (GOS 2-5) | 350 (48.27) |
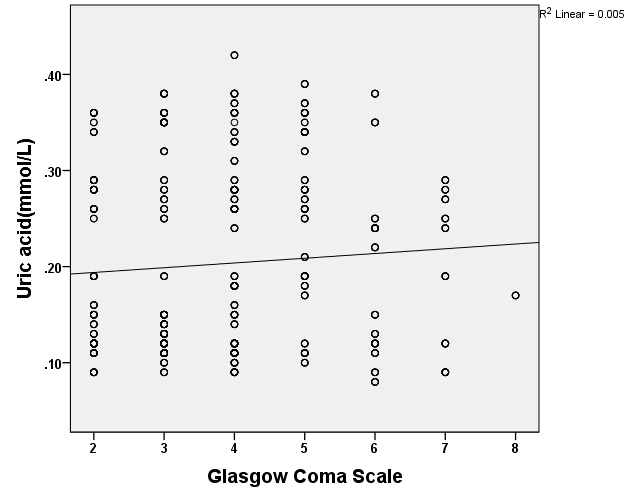
[Table/Fig-2] shows the association between UA levels and measures of GCS in TBI patients. There was a linear relationship between changes in the UA levels and GCS score. Thus, it can be postulated that elevated UA levels are associated with increased GCS score or the level of consciousness. In other words, there was a significant increase in the UA level and a corresponding improved consciousness in patients according to the GCS scores.
Relationship between uric acid levels and their glasgow coma score in patients with traumatic brain injury. Data are expressed as mean±SD, T-test analysis (p=0.005).
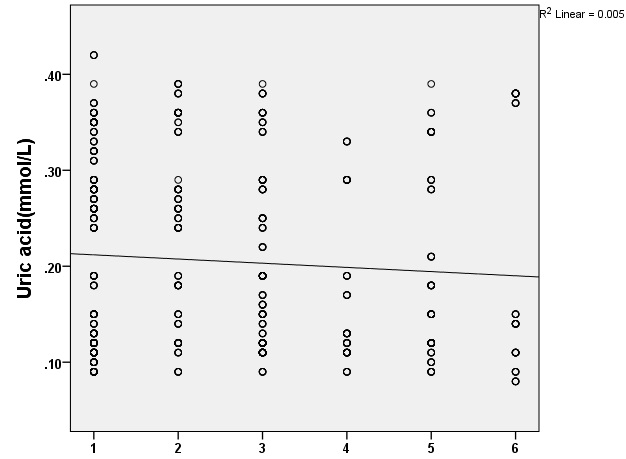
[Table/Fig-3] shows an inverse correlation between serum UA levels and the severity of brain injury by Marshall’s score. In other words, more the decrease in serum UA levels in TBI patients, the higher is the severity of brain injuries assessed by Marshall’s scores in TBI patients. Thus, there was a significant reverse relationship between UA levels and the severity of brain injury in TBI patients (p=0.005).
Relationship between uric acid plasma levels and classification of marshall’s score in patients with traumatic brain injury (1=Diffuse I, 2=Diffuse II, 3=Diffuse III, 4=Diffuse IV, 5= Evacuated Focal V, 6=Focal VI. T-test analysis, p=0.005).
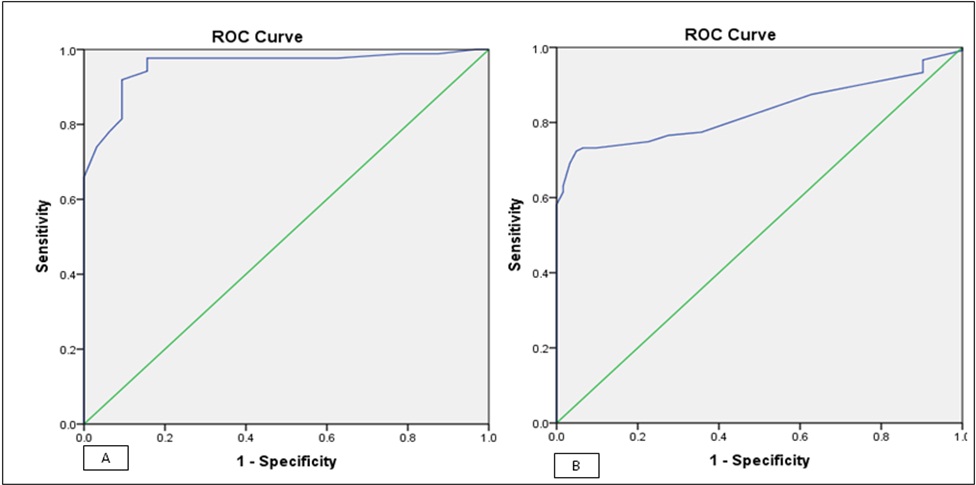
[Table/Fig-4] shows the sensitivity and specificity values of UA in determining in-hospital and six-month post-discharge outcomes. The UA level for in-hospital outcome was higher than that for the six-month outcome. The area under the curve by ROC curve was 0.830 (CI%95: 0.810-0.859) for in-hospital outcome and 0.957 (95% CI: 0.417-0.937) for six-month outcome. This result shows that the serum UA level had a higher sensitivity to determine the six-month outcome when compared with in-hospital outcome [Table/Fig-5].
Receiver-Operator Characteristic (ROC) analysis of acid uric for two outcomes.
| Variable | ROC | Asymptotic 95% Confidence Interval | Asymptotic Sig |
|---|
| Lower Bound | Upper Bound |
|---|
| In-hospital outcome | 0.830 | 0.810 | 0.859 | 0.000 |
| 6 month outcome | 0.957 | 0.417 | 0.937 | 0.000 |
Receiver-operator characteristic curves analysis of uric acid for the patients’ outcome: (a) receiver-operator characteristic of uric acid for in-hospital outcome (alive vs. dead); (b) Receiver-operator characteristic of uric acid for 6-month outcome (dead vs. alive).
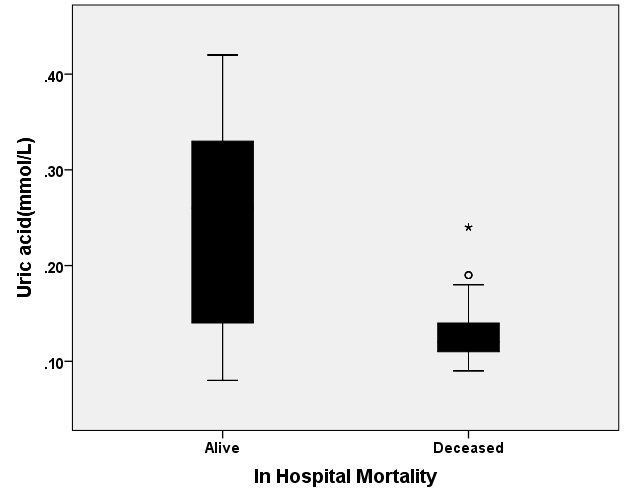
[Table/Fig-6] shows the relationship between the mean UA level and in-hospital prognosis (dead or survived) among TBI patients. The patients who died during their hospitalization showed significantly lower mean UA levels when compared with those who survived during hospitalization (0.126±0.026 vs. 0.243±0.942 mmol/l, p<0.001).
Relationship between the uric acid (mean±SD, mmol/l) level and in-hospital outcome in patients with traumatic brain injury, t-test analysis analysis (p=0.001).
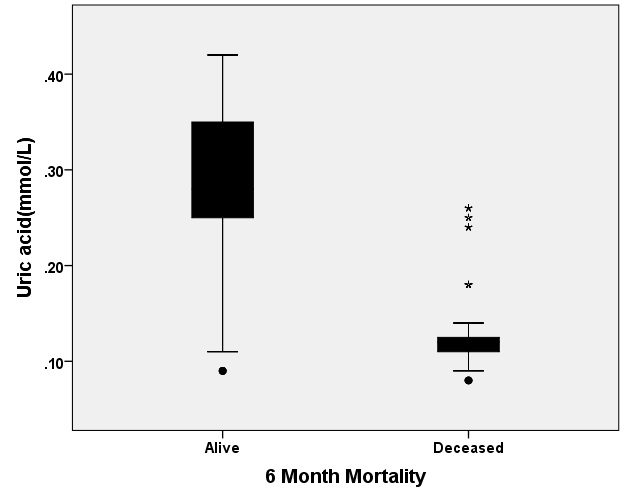
[Table/Fig-7] shows the relationship between the mean UA level and 6-month outcomes. As the figure shows, the mean UA level was lower in patients who died at six months after discharge when compared with that in patients who survived after six months discharge (0.130±0.044 vs. 0.286±0.069mmol/l, p<0.001).
Relationship between the uric acid (Mean±SD, mmol/L) level and 6 month outcome after discharged in patients with traumatic brain injury, t-test analysis analysis (p=0.030).
Discussion
The aim of our study was to investigate the relationship between serum UA levels and clinical characteristics such as level of consciousness, severity of brain injury and prognosis in patients with TBI. Nowadays, numerous studies have been conducted to investigate the level of various substances involved in oxidative processes in cell body and their associations with the clinical status of acute and chronic diseases of the CNS. Defined levels of free radicals responsible for oxidative processes play a central role in the cellular and molecular signaling for regulating the nature of neural and cellular function [22]. UA is the end product of purine metabolism and it has numerous antioxidant properties, including scavenging of free radicals such as hydroxyl, hydrogen peroxide and peroxynitrite; chelation of transition metals and inhibition of lipid peroxidation [23,24]. However, in addition to these antioxidant effects, UA may cause free radical activity in the xanthine oxidase pathway [25]. Previous studies have shown that assessment of the relation between specific markers and the cellular and molecular physiology of the brain is a good strategy for evaluating the prognosis and making appropriate decisions for management of TBI patients [26]. In this study, using a large sample size of participants, we examined the relationship between UA levels and the prognosis of TBI patients when in hospital and at 6 months after discharge. The majority of patients were females (57.10% vs. 42.89% men). The most common cause of TBI was vehicle crashes and the least common was gunshot injury. The majority of patients were classified as the diffuse type based on Marshall’s score of severity of brain damage, and only few cases were classified as focal mass lesion in the TBI patients. Among the 725 studied patients, the frequency of in-hospital mortality was 247 cases (34.06%) and 128 of the 478 (17.65%) discharged patients died during the six-month assessment. In the evaluation of UA levels, our study achieved interesting results about the relationship between UA levels and the prognosis of patients with TBI. For example, as shown by [Table/Fig-2], we observed a significant positive linear relationship between increase in serum UA levels and improvement in the levels of consciousness in patients assessed by the GCS scores. In other words, patients with higher levels of serum UA had a higher level of consciousness than those who had lower levels of UA. Our project is the first study indicating an association between increased levels of UA and improvement in the level of consciousness in TBI patients. In other diseases such as Parkinson’s, high levels of UA were associated with low incidence of the disease and good prognosis [27–30]. Also, patients with Multiple Sclerosis (MS) had lower levels of UA when compared with healthy subjects, and high UA levels in MS were associated with delayed onset of neurological episodes [31]. Improvement in the prognosis of Alzheimer’s disease was associated with high UA levels in many studies [32].
Our study is the first report to show a linear correlation between the UA levels and level of consciousness. Our results also showed a direct relationship between the severities of brain injury, as classified by Marshall’s score and UA levels; thus, it can be said that, greater the severity of brain damage, the lower is the serum level of UA. However, Langemann and colleagues showed that patients with brain trauma who died had high levels of serum UA compared with survived patients [1]. As our study and previous studies vary in terms of method and duration, the results cannot be directly compared. However, considerably higher levels of UA have been reported in early brain damages both in-vitro and in-vivo studies. Another important finding of our study was the inverse correlation between the severity of brain injury, on CT-scan findings, and serum UA levels. That is, with increasing the severity of brain damage, assessed by the Marshall’s score, the serum UA levels decreased significantly. Our study is the first to report this finding in a large sample of TBI patients under precise control of inclusion and exclusion criteria for patients’ enrollment and factors affecting the UA level. However, TBI-induced rat studies showed a direct association between the severity of brain damage and increasing the serum UA level, which was a finding in contrast to our results [33]. We also examined the relationship between patients’ prognosis and serum UA levels during hospitalization and six months after discharge. We found a significant relationship between serum UA level and survival either in hospital or at 6 months after discharge, a finding that was not reported by any study. In fact, it can be said that the serum UA levels could significantly predict the prognosis of patients with TBI. The ROC statistical analysis also showed that the area under the curve for prognosis in hospital was 0.830 and that for prognosis at 6 months after discharge was 0.957, suggesting that the UA level is strongly associated with prognosis at six months after discharge. In general, our study, which used an appropriate methodology with a large sample size, is the first to specifically report new results about the correlation between levels of UA in hospital and six months after discharge and clinical characteristics of TBI such as level of consciousness and the severity of brain damage based on CT-scan.
Limitation
One of the limitation of our study was the lack of information about the baseline level of UA in patients before the traumatic brain injury. In addition, we did not measure the serial levels of uric acid or levels after six months.
Conclusion
Despite limitations, we can say that the serum UA level can be a potential marker useful for managing TBI patients. Since, our study showed a relationship between UA levels and long-term prognosis of patients with TBI, it seems that monitoring the level of UA would be useful in the follow-up of TBI patients.
List of Abbreviations
UA: Uric Acid
TBI: Traumatic Brain Injury
CT: Computed Tomography
GCS: Glasgow Coma Scale
GOS: Glasgow Outcome Scale
CNS: Central Nervous System
ROC: Receiver-Operator Characteristic
ICU: Intensive Care Unit
MS: Multiple Sclerosis
Conflict of Interests: The authors do not have any actual or potential conflicts of interest in this study.
Funding/Support: This study was supported by Faculty of Medicine, Ilam University of Medical Sciences.