Neoplastic hilar obstruction to the liver outflow presents a unique challenge to the surgeon, wherein, the balance between a curative and possibly larger resection has to be achieved against a more conservative local resection. These are often technically demanding and have thus, far produced equivocal outcomes on both ends.
The present case series is on 13 patients who presented with hilar obstruction. They all underwent resections with possible curative intent. The focus of our review is on the technical nuances and the strategies we used, intra- and peri-operatively to make resections possible in these patients, who at first look were deemed inoperable. Among the 13, 10 had hilar cholangiocarcinoma (CCA) while the others had a more benign diagnosis e.g., Hydatid disease. We did not encounter any peri-operative mortality in our series. Two of our patients had to be re-explored for intra-abdominal complications. Among the 13, we encountered two deaths. The rest of the patients are still on follow-up as of April 2016. Hilar CCA continue to be rare and challenging tumours for the Hepato Pancreato Biliary (HPB) surgeon to manage. Outlooks are currently changing as we try to resect bigger and more complicated hilar liver tumours with better results.
Case Report
Patients who presented to us with obstructive jaundice secondary to hilar obstruction over the last two years (from 1st March 2014 to 31st January 2016) were retrospectively analysed. Institutional ethics committee clearance was obtained for the purpose of data collection.
The case details were collected on a case by case basis by the operating surgeon and the Hepato Pancreato Biliary (HPB) and transplantation team at the institution. The clinical case history and detailed examination findings were noted. This was followed up chronologically till April 2016 or till they were lost to follow-up or death. Most of the patients are still on follow-up. The documents included for data collection were the imaging findings, interventional procedures, neo-adjuvant therapy and any other clinical events of significance till resection. The intraoperative findings were reviewed along with the intraoperative notes, photographs and videos. The nomenclature of the resection procedures was based on the classification system proposed by Couinaud [1]. The postoperative course was followed-up similarly and the patients were followed-up till April 2016. The salient features of the clinical course of each patient are outlined in detail. The relevant events are then discussed along with a review of literature.
We reviewed a total of 13 patients with hilar obstruction. The demographics of the patients are included in [Table/Fig-1], along with the final diagnosis and treatment details of each patient. The predominant diagnosis in our series was that of cholangiocarcinoma (CCA).
Demographics, diagnosis and treatment details.
| Sl.No. | Age(year) | Sex | Diagnosis | Co-morbidities | Specialtumourfeatures | Operativeprocedure | Histopathology- (Differentiation/LVI/PNI/Margins) | Adjuvant /NeoadjuvantTherapy (A/N) | Radiationtherapy | AssociatedFigure |
|---|
| 1 | 35 | F | Hilar CCA | Hypothyroidism | Segment4 extension | Righttrisegmentectomy+ Left HJ | Well/ + / + / clear | GemOx (A) | Nil | [Table/Fig-2] |
| 2 | 50 | M | Left lobe CCA | Nil | Hilar extensionwith tumourthrombusextending toCBD | Left Hepatectomy+ Right HJ | poor/ + / + / clear | GemOx (A) | Nil | [Table/Fig-3] |
| 3 | 43 | M | Hilar CCA | Nil | Left lobeextension | Left Hepatectomy+ Right HJ | moderate/- / - / involved | GemOx (A) | Brachytherapy | Nil |
| 4 | 49 | M | Hilar CCA | Type 2 DiabetesMellitus | Left lobeextension | Left Hepatectomy+ Right HJ | poor/ + /+ / involved | Gem+Cisplatin (N) | IMRT | [Table/Fig-4] |
| 5 | 36 | M | Hilar CCA | Nil | RepeatedCholangitishistory | Dome resection+ Multiple HJ | moderate/ - /- / clear | Refused | Nil | Nil |
| 6 | 65 | F | Hilar CCA | Nil | Nil | Dome resection+ Multiple HJ | Well / - /- / clear | Refused | Nil | Nil |
| 7 | 47 | M | Low gradeneuroendocrinetumour of CBD | Nil | Benign BileductTumour | CBD Resectionwith HJ | Benign | NotApplicable | NotApplicable | Nil |
| 8 | 37 | M | Left hepatic ductcystadenoma+ RupturedHydatid cyst | Nil | Coexistenthydatidcyst rupturecausingbiliary obstruction | Left LateralSegmentectomy+ CBD Exploration | Benign | NotApplicable | NotApplicable | [Table/Fig-5,6] |
| 9 | 54 | F | Papillary neoplasmof Left lobe liver withCBD Communication | Nil | Benign Bileduct Tumour+ CBDCommunication | Left Hepatectomy+ Right HJ | Benign | NotApplicable | NotApplicable | Nil |
| 10 | 52 | M | Hilar CCA | Nil | Left lobe andCBD extension | Left Hepatectomy+ Right HJ | moderate / -/ - / clear | GemOx (A) | IMRT | Nil |
| 11 | 75 | F | Hilar CCA | Nil | Left lobeextension | Left Hepatectomy+ Right HJ | moderate /- / - / clear | GemOx (A)-Plan | IMRT | Nil |
| 12 | 53 | F | Hilar CCA | Nil | Right lobeextension | Right Hetapectomy+ Left HJ | poor / + /+ / clear | GemOx (A)-Plan | IMRT | Nil |
| 13 | 50 | M | Hilar CCA | Nil | Left Lobeextension+ Cholangitis | Left Hepatectomy+ Right HJ | poor / + /+ / clear | GemOx (A)-Plan | IMRT-Plan | Nil |
Footnote: CCA-cholangiocarcinoma; CHD-Choronary Heart Disease; CBD- Common Bile Duct; HJ- Hepaticojejunostomy; IMRT- Intensity Modulated Radiation Therapy
Case-1
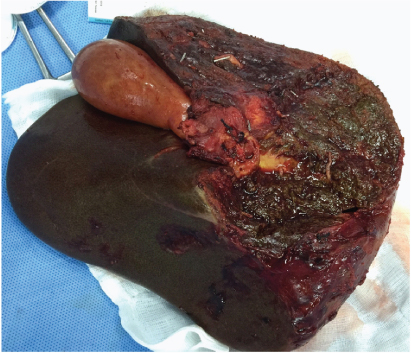
In addition to the features given in the table, this patient also had contiguous infiltration of main portal vein and encasement of right branch of common hepatic artery. This is what led us to the decision of performing a right trisegmentectomy after assessment of the adequacy of the remnant liver volume [Table/Fig-2].
Resected specimen in Case 1. She underwent a right trisegmentectomy.
Case-2
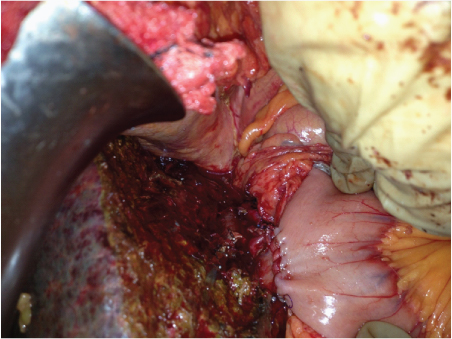
In the fourth month postoperatively, he was found to have a segment V nodule that was active on PET-CT in the setting of a stable CA19-9. It was resected and found to be negative for malignancy. However, two months later, he was found to have diffuse omental nodularity in the setting of a rising CA19-9 titre. He was switched over to palliative chemotherapy with paclitaxel and capecitabine. He is currently on palliative chemotherapy and has stable disease on further imaging [Table/Fig-3].
Right hepaticojejunostomy performed after a left hepatectomy, Case-2.
Case 3
His preoperative course was significant in that he underwent a Percutaneous Transhepatic Biliary Drainage (PTBD) and drainage with a covered metallic stent. The PTBD catheter was found to be blocked and subsequently underwent Endoscopic Retrograde Cholangio-pancreatography (ERCP) and stenting for the same. His malignancy was found to involve the secondary ducts on the left side thus, necessitating a resection for the resolution of obstructive jaundice. The possible palliative nature of the surgery and the need for adjuvant therapy were discussed with the patient and his family pre-operatively. The surgical margins were positive (microscopic) at the site of the duct with the covered stent. He received brachytherapy through the PTBD catheter. The patient however, due to logistical issues, delayed further adjuvant therapy by a period of four months, during which he developed cholangitis with multiple abscesses in the liver remnant. This was treated conservatively. Upon resolution of the cholangitis, he received palliative GemOx which he tolerated for four cycles before being documented with recurrence in the peritoneum. He was since then managed on palliative therapy and died in the eighth month post-op.
Case 4
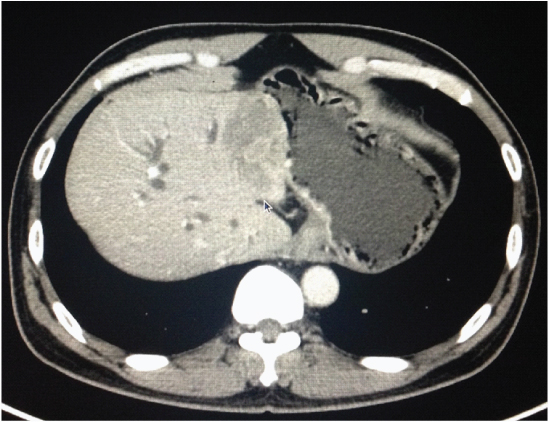
He was presented to another centre with obstructive jaundice and had undergone ERCP and stenting for the same. Imaging revealed a hilar lesion with left lobe involvement, with the secondary ducts also being affected [Table/Fig-4]. A PTBD and stenting with a covered metallic stent was further done to drain the right lobe and palliate the obstructive jaundice. He developed recurrence in the peritoneum and omentum in the sixth month post-op, was started on palliative GemOx. He tolerated three cycles of chemotherapy before succumbing in the ninth month after surgery.
Hilar mass with left lobe involvement, Case-4.
Case 5
This patient presented to us after being labelled as inoperable CCA on an exploratory laparotomy at a university hospital outside. He had also undergone ERCP and stenting at a local hospital for the same. Imaging revealed possibly neoplastic thickening and narrowing of the bile ducts at the hilum. There was significant associated lymphadenopathy at the porta. Reconstruction in this patient was done with three hepaticojejunostomies (two right and one left) to a roux loop of jejunum. Postoperatively, his course was complicated by haemorrhage into the drain which necessitated re-exploration. It was noted to be from the jejunalmesentry and was dealt with appropriately.
Case 6
This patient underwent a resection similar to case 5 with a segmental resection of segments IV and V, a portal lymphadenectomy and two hepaticojejunostomies (one right and one left). She is currently in her fourth month postoperatively and has thus far shown no evidence of recurrence on an abdominal CT scan.
Case 7
This patient presented with a two-year history of abdominal pain preceding a two-month history of obstructive jaundice. He was diagnosed to have a tumour in the Common Bile Duct (CBD) that had grown and obstructed the hilum after imaging.
Case 8
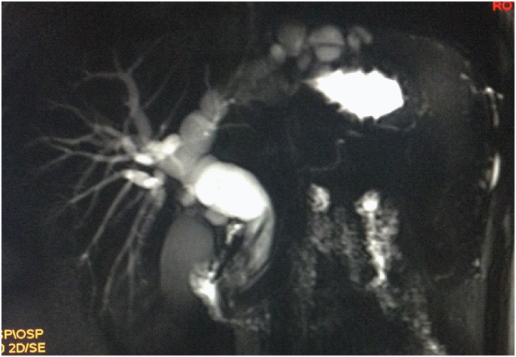
This patient presented to us with a gradually progressive abdominal pain with sudden onset obstructive jaundice. His MRI showed an ill defined lesion in segments II and III with gross dilatation of the ductal system [Table/Fig-5]. A presumptive diagnosis of intra-biliary rupture of hydatid cyst was made. Intra-operatively, he had a grossly dilated CBD, which when explored and decompressed, led to the regression of the left lateral segment lesion upon draining of clear fluid [Table/Fig-6].
MRI showing complex left lobe cystic lesion with CBD communication, Case-8.
Hydatid cyst left lobe with CBD communication. Case-8.
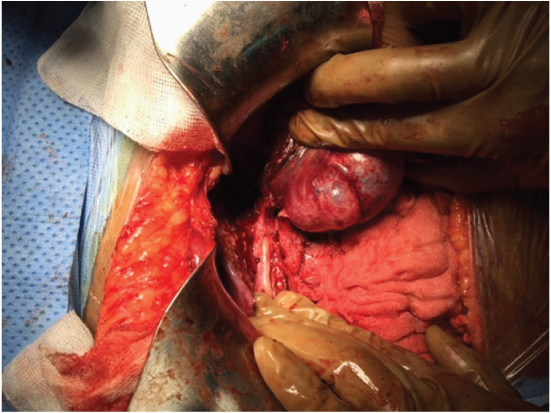
Case 9
Imaging studies on this patient revealed a complex cystic lesion in segments II and III and there was apparent communication noted between the lesion and the left hepatic duct. Consequent atrophy of the left lobe of the liver was also noted on the CT.
Case 10
In this patient, celiac lymphadenectomy was also performed concurrently due to enlarged nodes found at operation.
Case 11
This patient’s postoperative course was complicated by basal atelectasis and pneumonia which needed management with non-invasive ventilator support and led to a prolonged hospital stay.
Case 12
She presented to us after being diagnosed to have a hilar mass at operation during a laparoscopic (converted to open) cholecystectomy for gall stone disease. She subsequently underwent ERCP and stenting. Imaging revealed right sided extension of the hilar mass and was diagnosed to have hilar CCA with right sided extension.
Case 13
This 50-year-old male presented to us in a state of sepsis. He was diagnosed to have hilar obstruction secondary to a hilar mass for which ERCP and stenting had been done. Subsequently he had developed cholangitis and sepsis with acute kidney injury. He was resuscitated and managed with intravenous antibiotics for the same. His imaging revealed a hilar mass with left lobe infiltration.
Discussion
Malignancy at the confluence of the right and left hepatic ducts were first described by Altmeier in 1957 and has been described by the eponym Klatskin tumour after his landmark series in 1965 [2,3]. Although CCAs may arise anywhere in the biliary tree, the hilar location is the most frequently affected accounting for 60-80% of all CCA [4]. CCAs are rare cancers, constituting less than two percent of all human cancers [5]. The anatomy at the hilum, their location in the hepatoduodenal ligament, proximity to vascular structures and extension into the liver make CCAs difficult to characterize before and during surgery. Regional or hepatic metastases are seen in up to 50% of cases at presentation [6].
Almost all cases present with obstructive jaundice. There may be additional symptoms of loss of appetite or weight loss. Pain and features of sepsis may be prominent in the setting of cholangitis, which usually occurs after biliary stenting.
Early attempts at resection were by Altmeier et al., and most tumours then were intubated when discovered at laparotomy. They noted that most right sided tumours were considered intra-hepatic or unresectable at the time. They also commented that with a right sided tumour, the length of left sided duct available for a reliable hepaticojejunostomy was better and possibly allowed for better outcomes in those who did undergo resections [2].
Imaging these patients cannot yet reliably demonstrate the tumour and, literature is strewn with multiple reports of lesions that mimic Klatskin tumours in every aspect. This “malignant masquerade” at the liver hilum ranges from 5-15% in most series with some reporting absence of malignancy in upto a third of their resection specimens [7–11].
There are three main morphological variants of CCA: Infiltrating, Exophytic and Polypoidal. Infiltrating is the most common variety of hilar CCA that appears as focal thickening of the bile duct on imaging. The involvement of secondary ducts, vascular invasion, increased duct wall thickness etc., may point to a diagnosis of CCA but are not definite. The presence of hilar lymphadenopathy in the setting of inflammatory or infectious diseases like sarcoidosis or tuberculosis may also lead to a misdiagnosis of advanced CCA [12].
Although, a definite pre-operative diagnosis is desirable, it is not possible to achieve in all cases. Newer modalities such as a cholangioscopy with cytology hold promise and are under evaluation currently [13,14]. Nonetheless, most of these patients require surgical intervention (in the form of major resections) for relieving the obstructive jaundice (with curative intent if feasible). Tumour markers like CA19-9 or IL-6, although sufficiently raised in CCA, lack the power to distinguish between benign and malignant hilar lesions [15,16].
Cystic lesions at the hilum may also lead to obstruction with jaundice. In these cases, it is necessary to rule out an exophytic variety of intra-hepatic CCA. Hydatid cysts may also occasionally present with obstructive jaundice occasionally secondary to hilar obstruction. This may be extra-luminal due to a hydatid cyst that compresses the biliary tract or one that has ruptured into the biliary tract with intra-luminal obstruction. A rarer cause of hilar obstruction is a biliary cystadenoma compresseing the ductal system at the confluence [12].
In our series, we found that, 12 of the 13 patients have CCAs causing hilar obstruction. Of these, 11 were hilar CCAs and one was intra-hepatic CCA with extension of tumour thrombus to the hilum and upto the suprapancreatic CBD. This correlates with reports in literature wherein the most common location of CCA was hilar [4,17].
Patients present with high bilirubin levels. Surgery is the only curative option often available to these patients. If not feasible, interventional procedures combined with palliative surgical options must be thought of with an aim to improve quality of life. Interventional procedures in hilar tumours should not be attempted if curative resection is possible. Interventions through the tumour has been found to cause tumour spillage (leading to higher recurrence rates), cholangitis and may alter the stage of the disease [18,19].
Each case poses a unique challenge. Careful assessment of the biliary system on imaging is the key to decision making. A dedicated radiologist who attends the tumour board on a regular basis will help guide the decisions. Interventional procedures or ERCP should be used only when necessary and avoided in patients capable of undergoing surgery with curative intent. In the present case series, we found that, nodal status was positive in patients who underwent interventions to relieve jaundice as against patients who underwent surgery straight away.
Vascular involvement is another important prognostic factor. Major or minor vascular involvements are poor prognostic indicators by themselves [11]. Minor involvement being a pathological indicator is ascertained after surgery. Major involvement of either the right or the left hepatic arteries or the portal veins by direct invasion have to be dealt with at the time of surgery. Hepatic artery or the portal vein that are involved have been described to be dissected, or at times a segment resected and anastomosed, and or a prosthetic material being interposed. We believe that when there is involvement of right or left lobe major vessels, performing lobectomy along with the vascular component might improve the overall prognosis by virtue of obtaining greater disease clearance margin. In cases with major vascular involvement, options may be limited to an extended hemi-hepatectomy (e.g., right trisegmentectomy) with dissection through the tumour at the hilum necessary for obtaining vascular clearance. In such cases, it is preferred to perform the ALPPS (Associating Liver Partition with portal vein ligation for Staged hepatectomy) procedure rather than dissecting through the tumour for obtaining vascular clearance [20].
Lobectomy also addresses the tumour infiltration to the periductual area and the ipsilateral vascular structures to a certain extent. Most patients would have had an intervention to relieve obstruction at an outside facility and instrumentation has been found to enhance tumour progression. Lobectomy handles the local progression of the tumour in such situations [21].
Right or left lobe resection with remnant hepaticojejunostomy gives us a margin free of tumour. This may not be possible in all cases. Making sure the secondary ducts are not involved in a hilar CCA on the remnant side, and stenting (covered stent) the same was discussed when no other option was viable. This was followed by resection of the involved lobe and the tumour at the hilum over the covered metallic stent. The resection status was addressed postoperatively with external beam or brachytherapy and chemotherapy. In such cases, we found that resection was the only measure that could improve the jaundice to such levels that the patient could tolerate adjuvant therapy at the earliest. Palliative stenting alone, in such a patient, can also lead to florid cholangitis and the therapy may not proceed thus [22].
Conclusion
The management of patients with malignant hilar obstruction is a field where still much remains to be achieved. Surgery is the only modality that offers any hope of long term survival in patients with CCA when performed to negative margins. Surgery often is complicated by the location and size of the tumour, which may warrant major hepatic resection or if necessary, a transplant. Patients with CCA often have multiple co-morbidities and thus may be unable to tolerate extensive hepatic resections. This is also confounded by issues such as the use of pre-operative chemotherapy or cholangitis resulting often as a complication of a biliary stent.
Footnote: CCA-cholangiocarcinoma; CHD-Choronary Heart Disease; CBD- Common Bile Duct; HJ- Hepaticojejunostomy; IMRT- Intensity Modulated Radiation Therapy
[1]. Couinaud C, Etude anatomiques et chirugicales 1957 ParisMasson:400-409. [Google Scholar]
[2]. Altemeier WA, Gall EA, Zinninger MM, Hoxworth PI, Sclerosing carcinoma of the major intrahepatic bile ductsArch Surg 1957 75:450-60. [Google Scholar]
[3]. Klatskin G, Adenocarcinoma ofthe hepatic duct at its bifurcation within the porta hepatisAm J Surg 1965 38:241-56. [Google Scholar]
[4]. Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD, CholangiocarcinomaThe Lancet 2005 366(9493):1303-14. [Google Scholar]
[5]. Meining A, Chen YK, Pleskow D, Stevens P, Shah RJ, Chuttani R, Direct visualization of indeterminate pancreaticobiliary strictures with probe-based confocal laser endomicroscopy: a multicenter experienceGastrointestinal Endoscopy 2011 74:961-68. [Google Scholar]
[6]. Blumgart LH, Benjamin IS, Cancer of the bile ducts. In Blumgart LH, edSurgery of the liver and biliary tract 1994 2nd edNew YorkChurchill Livingstone:967-996. [Google Scholar]
[7]. Wetter LA, Ring EJ, Pellegrini CA, Way LW, Differential diagnosis of sclerosingcholangiocarcinomas of the common hepatic duct (Klatskintumours)Am J Surg 1991 161:57-63. [Google Scholar]
[8]. Verbeek PC, van Leeuwen DJ, de Wit LT, Reeders JW, Smits NJ, Bosma A, Benign fibrosing disease at the hepatic confluence mimicking KlatskintumoursSurgery 1992 112:866-71. [Google Scholar]
[9]. Gerhards MF, Vos P, van Gulik TM, Rauws EA, Bosma A, Gouma DJ, Incidence of benign lesions in patients resected for suspicious hilar obstructionBr J Surg 2001 88:48-51. [Google Scholar]
[10]. Knoefel WT, Prenzel KL, Peiper M, Hosch SB, Gundlach M, Eisenberger CF, Klatskintumours and Klatskin mimicking lesions of the biliary treeEur J Surg Onc 2003 29:658-61. [Google Scholar]
[11]. Kimura N, Young A, Toyoki Y, Wyatt J, Toogood G, Hidalgo E, Radical surgery for hilar cholangiocarcinoma in comparable eastern and western centers – outcome analysis and prognostic factorsHPB 2016 18:e68-69. [Google Scholar]
[12]. Koea J, Holden A, Chau K, McCall J, Differential diagnosis of stenosing lesions at the hepatic hilusW J Surg 2004 28:466-70. [Google Scholar]
[13]. Leelawat K, Narong S, Wannaprasert J, Leelawat S, Serum NGAL to clinically distinguish cholangiocarcinoma from benign biliary tract diseasesInt J Hepatology 2011 2011:8735486 pages [Google Scholar]
[14]. Chen YK, Shah RJ, Pleskow DK, Chuttani R, Silvika A, Stevens PD, Miami classification (MC) of probe-based confocal laser endomicroscopy (pCLE) findings in the pancreaticobiliary (PB) system for evaluation of indeterminate strictures: interim results from an international multicenter registryGastrointestinal Endo 2010 71(5):AB134 [Google Scholar]
[15]. Ascenti G, Scribano E, Loria G, Vallone A, Pandolfo I, Gaeta M, Computerized tomography in the assessment of obstructive jaundice caused by hepatic hydatid cystsLa Radiologia Medica 1995 89(6):804-08. [Google Scholar]
[16]. Nemati-Honar B, Hayatollah G, Nikshoar M, Forootan M, Feizi A, Liver hydatid cyst and acute cholangitis: a case reportActa MedicaIranica 2016 54(4):286-88. [Google Scholar]
[17]. Ruys AT, Van Beem BE, Engelbrecht MRW, Bipat S, Stoker J, Van Gulik TM, Radiological staging in patients with hilar cholangiocarcinoma: a systematic review and meta-analysisThe British Journal of Radiology 2012 85(1017):1255-62. [Google Scholar]
[18]. Nennstiel S, Weber A, Frick G, Haller B, Meining A, Schmid RM, Neu B, Drainage-related complications in percutaneous transhepatic biliary drainage: an analysis over 10 yearsJ Clin Gastroenterol 2015 49(9):764-70. [Google Scholar]
[19]. Takahashi Y, Nagino M, Nishio H, Ebata T, Igami T, Nimura Y, Percutaneous transhepatic biliary drainage catheter tract recurrence in cholangiocarcinomaBr J Surg 2010 97:1860-66. [Google Scholar]
[20]. de Santibañes, Eduardo MD, Clavien PA, Playing play-doh to prevent postoperative liver failure: The “ALPPS” approachAnnals of Surgery 2012 255(3):415-17. [Google Scholar]
[21]. Neuhaus P, Thelen A, Jonas S, Puhl G, Denecke T, Veltzke-Schlieker W, Oncological superiority of hilar en bloc resection for the treatment of hilar cholangiocarcinomaAnn Surg Oncol 2012 19(5):1602-08. [Google Scholar]
[22]. Walter T, Ho CS, Horgan AM, Warkentin A, Gallinger S, Greig PD, Endoscopic or percutaneous biliary drainage for Klatskintumours?J VascInterv Radiol 2013 24(1):113-21. [Google Scholar]