Chronic Obstructive Pulmonary Disease (COPD) is a major cause of disability and death, all over the world. India has a share of approximately 5,56,000 case of COPD (>20%) and 1,354,000 cases are from China (about 50%), out of a world total of 2,748,000 annually [1]. India contributes a significant percentage of mortality of COPD. A total of 27,756 thousand Disability Adjusted Life Years (DALYs) are contributed due to COPD globally and 6,740 thousand DALYs are from India, indicating significant impact on health related Quality of Life (QoL) globally as well as in India [2]. According to Global Initiative against Obstructive Lung Diseases (GOLD 2015) definition, COPD is a preventable and treatable disease, characterised by persistent airflow limitation which is progressive, with exacerbations and extra pulmonary components contributing to overall severity of the disease [3]. COPD impairs QoL, by preventing people with the condition from socializing and enjoying their hobbies [4]. Poor physical performance results due to dyspnoea, anxiety of dyspnoea, de-conditioning and limited mobility (vicious cycle) eventually resulting in poor QoL. Acute exacerbation contributes to diminished health related QoL [5].
In general, medical professionals have nihilistic approach towards COPD. QoL is an important domain for measuring the impact of chronic disease. Both general and disease-specific instruments have been used to measure QoL in patients with COPD [4]. Health care use by COPD patients appear to be related more to an impaired QoL than severity of COPD by itself [6], therefore, improvement in QoL should be one of the aims in treatment of COPD [7].
Tobacco smoking is a major risk factor of COPD. Cigarette smoke contains numerous free radicals and other oxidants that may induce an oxidant/anti-oxidant imbalance within the lung in smokers [8]. There is evidence that, endogenous oxidants, such as reduced Glutathione (GSH) and GSH bio-synthezing enzymes are significantly decreased in these patients [9]. There is a large body of evidence that, an increase in oxidative stress plays an important role in the pathogenesis of COPD [10]. Oxidative stress has been reported to increase and GSH levels are depleted during severe COPD exacerbations [11]. N-Acetylcysteine (NAC) is a precursor of GSH and both these agents act as a free radical scavenger [8]. It is estimated that 25-45% patients with COPD have never smoked [12]. Apart from tobacco smoking, COPD is associated with biomass, occupational exposure to dusts and gases, history of pulmonary tuberculosis, chronic asthma, respiratory infections during childhood, outdoor air pollution and poor socio-economic status [12].
Health Related QoL (HRQoL) relates well to exercise performance, mobility and dyspnea too relates well with HRQoL, than Forced Expiratory Volume in first second (FEV1) [5]. One study shows that, moderate to high levels of regular physical activity is associated with a lower lung function decline and risk of COPD in active smokers [13] and low levels of physical activity predict all-cause of mortality in patients with COPD [14]. Many physicians advise COPD patients, to exercise at home (walking for 20 minutes) for those who cannot participate in structured rehabilitation program (GOLD). The benefit of this general advice has not been tested but observational studies have indicated several benefits [3]. There is very little COPD specific evidence to support recommendation for physical activity other than studies of pulmonary rehabilitation [3]. The present study was done to study the combined effect of administration of 600mg of NAC once daily and 20 minute walk as daily physical activity for ten weeks in addition to standard treatment as compared with control group (placebo and standard treatment) on QoL in stable COPD patients. The tool that we used to measure HRQoL was Saint George Respiratory Questionnaire-C (SGRQ-C) (COPD specific) [15].
Materials and Methods
This was randomised, parallel group, open labelled, placebo controlled trial. The study was conducted at ESI Postgraduate Institute of Medical Sciences and Research MGM Hospital, Parel, Mumbai from December 2011-December 2013. After obtaining approval from Institutional Ethics Committee (IEC), 100 patients of COPD were enrolled in the study as diagnosed according to GOLD guidelines. Written and informed consent was obtained from each of the selected patients.
Inclusion criteria: Patients diagnosed as stable COPD on basis of FEV 1 according to (GOLD guidelines 2010) of age 40 years to 70 years.
Exclusion criteria: 1) Elderly patients above 70 years; 2) Pregnant patients; 3) Patients in acute exacerbation at the time of enrolment and history of exacerbation in last 6 weeks; 4) Patients with severe ischemic heart disease, recent history of Myocardial Infarction (MI), stroke, severe hypertension (systolic>180 and diastolic >110), pulse>120; 5) Patients unable to walk the predicted distance for their age in six minutes’ walk test.
Basic demographic data of all patients was collected. Patient’s occupation, exposure to smoke (active or passive), fumes, allergens, agents causing occupational exposure were noted. Patients were enquired about respiratory symptom and history of smoking, past history of pulmonary tuberculosis, childhood infection i.e., measles, pneumonia, history of exposure to wood-chulha, indoor air pollution, history of diabetes, systemic hypertension, family history of bronchial asthma and atopy and other relevant history was noted. After complete physical examination, patients were subjected for following investigations: chest X-ray view, pulmonary function test, ECG, complete haemogram, liver function test, renal function test, six minute walk test.
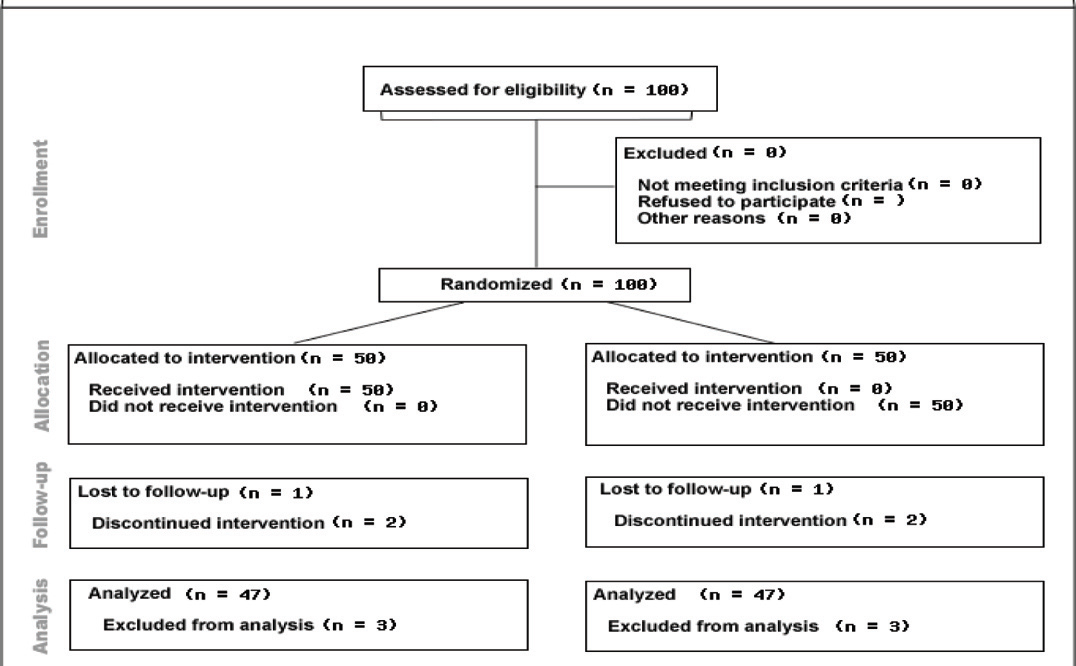
Patients were divided as study subjects (receiving NAC and 20 minute walk as home based exercise and standard treatment) and controls (receiving placebo and standard treatment) randomly. Odd and even numbers were allotted for study group and control patients respectively as and when they attended the Chest OPD. SGRQ-C (translated in local languages they understand) was administered to all patients [Table/Fig-1]. The patients selected in study group, were given active drug, NAC 600mg (effervescent tablet) once daily in morning 15 minutes after breakfast, dissolved in a glass of water (in addition to their regular treatment) and simultaneously instructed to walk for 20 minutes daily, at their own pace as home based exercise. The study group patients were provided charts individually to maintain the record of their daily physical activities (walk for 20 minutes). This regimen was prescribed for 10 weeks. The patients selected as controls were given placebo once daily in morning 15 minutes after breakfast dissolved in a glass of water along with their standard therapy for 10 weeks and to continue their routine daily activities. They were followed-up weekly to check for their adherence to the treatment. After completion of ten weeks, SGRQ-C was re-administered to all the patients who continued to be in the study. Score of SGRQ-C before and after the completion of 10 weeks was calculated and compared. The SGRQ-C includes three components: Symptoms; Activity and Impact. The difference of 4 in the score was considered significant.
Each component of the questionnaire was scored separately. Symptoms include questions 1 to 7, Activity include questions 9 and 12 while Impact include questions 8, 10, 11, 13, 14. Then total score is calculated by summing the weights to all the positive responses in each component. Score = 100 x summed weights from all positive items in the questionnaire/sum of weights for all items in the questionnaire. Sum of maximum possible weights for each component and Total: Symptoms- 566.2; Activity-982.9; Impacts-1652.8; Total (sum of maximum for all three components) - 3201.9.
Statistical Analysis
The t–test was applied for statistical analysis of SGRQ-C score for study and control group. Pearson chi-square test was applied for comparison of study and control group (SGRQ-C score). Pearson correlation was used for relationship of SGRQ-C score and quality of life.
Results
Out of 100 patients enrolled in this study, there were 86 males and 14 females (43-males and 07 females in each group), showing equal distribution gender wise in ‘study’ and control group. It was observed that 90% of patients were between 51 to 70 years of age group. A 10% of patients were in the age group of 41 to 50 years. Mean age in study and control group was nearly the same [Table/Fig-2,3]. Six patients did not continue the study (3 i.e., 6% patients receiving drug and 3 i.e., 6% receiving placebo). Out of 6 patients, 3 patients from the study group did not continue the study because of taste of the drug whereas one patient from control group had similar reason for non-compliance or opting out of study, although the effervescent placebo tablet did not contain NAC. Two patients did not follow-up study for unknown reasons. Loss to follow-up was less than 10% [Table/Fig-1].
Baseline characteristics of patients enrolled in study.
| No. of Patients (n=100 patients –enrolled for the study) | n = 94No. of patients at the end of trial |
|---|
| AgeMean- 59.36S.D.-5.32 | 41-50 | 10 |
| 51-60 | 45 |
| 61-70 | 45 |
| Gender | Male | 86 |
| Female | 14 |
| BMI | Mean- 18.96S.D.-1.86 |
| Six Minute Walk Test (Distance) | Mean-373.66mS.D.-39.05 |
| H/O Pulmonary TB | 41 |
| Smoking Status | No. of Patients |
| Current Smoker | 9 |
| Former Smoker (Left since 1 yr) | 63 |
| Non-Smoker | 28 |
| GOLD Classification | No. of Patients |
| I | 17 |
| II | 10 |
| III | 61 |
| IV | 12 |
Gender wise distribution in study and control group.
| Study group | Control group |
|---|
| N | N % | N | N % |
|---|
| Gender | Male | 43 | 86.0% | 43 | 86.0% |
| Female | 7 | 14.0% | 7 | 14.0% |
| Total | 50 | 100.0% | 50 | 100.0% |
By applying Independent t-test, we observed that mean age in study group and control patients was nearly equal [Table/Fig-4].
Mean age of study and control groups.
| Type | N | Mean |
|---|
| Age | Cases | 50 | 59.44 |
| Control | 50 | 59.36 |
Twenty-one i.e., 44.7% patients from study group (who received active drug NAC and 20 minutes daily walk in addition to standard treatment) had significant change in the SGRQ-C score after 10 weeks compared with the initial score. A total of 26 i.e., 55.3% patients had insignificant difference of score after ten weeks compared to initial score (change in SGRQ-C score <4). The patients who were enrolled in control group, 11 i.e., 23.4% patients showed significant difference of score after 10 weeks compared with initial score and 36 i.e., 76.6% patients did not show significant difference of score at the end of ten weeks compared with initial score [Table/Fig-5].
SGRQ-C significance distribution between study and control group.
| Study group | Control group |
|---|
| N | N % | N | N % |
|---|
| SGRQ-C | >=4 | 21 | 44.7% | 11 | 23.4% |
| <4 | 26 | 55.3% | 36 | 76.6% |
| Total | 47 | 100.0% | 47 | 100.0% |
Paired t-test was applied to the study and control groups. There was significant improvement in the SGRQ-C score of study group (p-value= 0. 0001) as compared to control group (p-value= 0.118) [Table/Fig-6,7].
SGRQ-C score among study group (patients who received NAC and 20 minutes daily walk).
| N | Mean | Std. Deviation | p-value |
|---|
| Pre SGRQ- C | 47 | 38.54 | 9.04 | 0.0001 |
| Post SGRQ-C | 47 | 33.82 | 7.75 |
Paired t-test was applied.
SGRQ-C score in control group (patients who received placebo).
| N | Mean | Std. Deviation | p-value |
|---|
| Pre SGRQ-C | 47 | 39.16 | 8.547 | 0.118 |
| Post SGRQ-C | 47 | 37.82 | 7.79417 |
At the end of ten weeks, it was observed that mean change in SGRQ-C was significant in “study group” as compared to “control group”. Mean change in score-(Independent t-test) among study group was 4.72 and the same in control was 1.32, p-value - 0.007 [Table/Fig-8].
Mean change in SGRQ-C between study and control group.
| Type | N | Mean | Std. Deviation | p-value |
|---|
| Change in SGRQ-C | Study group | 47 | 4.7221 | 6.21903 | 0.007 |
| Control group | 47 | 1.3262 | 5.70592 |
Independent t – test was applied.
Independent t-test was applied to the study and control groups. There was significant improvement in the SGRQ-C score of study group (p-value= 0. 0001) as compared to control group (p-value- 0.118). The overall p-value by applying Pearson chi-square test was found to be significant (p-value= 0.03) [Table/Fig-9].
Pearson’s chi-square tests.
| Type |
|---|
| SGRQ-C | Chi-square | 4.738 |
| df | 1 |
| p-value | 0.03 |
Results are based on nonempty rows and columns in each innermost sub table.
* The Chi-square statistics is significant at the 0.05 level.
Discussion
QoL in a chronic condition such as COPD is an important aspect of management, as there are limitations of currently available treatment guidelines. In the current study, we have combined 600mg NAC and daily physical activity with placebo control (in addition to standard treatment) and found statistically significant improvement in QoL in the study group.
To our knowledge, combined effect of NAC and daily physical activity in stable COPD, on HRQoL has not been reported in the literature, hence, we reviewed a few out of available studies, about NAC in stable COPD and physical activity in stable COPD (as individual topics) but we could not compare our results with other studies.
Orally taken NAC is rapidly absorbed following oral administration, and is quickly metabolised to cystiene a direct precursor in the synthesis of intra-cellular GHS [16]. It has been shown that, oral administration of NAC 600mg/day for 5 days significantly increases GSH concentrations in the bronchoalveolar lavage fluid of treated individuals, as compared with those who did not receive NAC (p<0.05) 1–3 hours after the last dose of NAC [17]. NAC has been studied in various studies on oxidative stress, frequency of cough, on dyspnea, in number of exacerbations, effect on air trapping, and many studies have shown an impact on QoL in COPD directly or indirectly. In one study, both the doses (600mg OD and 600mg BID) of NAC had a good outcome on the oxidative stress, and other clinical symptoms like cough intensity and frequency [18].
A systematic review of Randomised Controlled Trials (RCTs) comparing NAC with placebo was undertaken to analyse the effects of NAC treatment on COPD exacerbations. This analysis, which considered 11 studies including 2011 patients, revealed that a greater number of patients were free of exacerbations during treatment with NAC 400-600mg/day for 3–6 months, as compared to patients treated with placebo. In particular, 351 out of 723 patients treated with NAC (48.5%) did not experience exacerbations during the treatment period, as compared with 229 out of 733 (31.2%) patients who received placebo. This difference was statistically significant (p<0.05) [19].
Some studies have shown benefits with higher dose of NAC in COPD. De Benedetto et al., reported in a randomised placebo controlled trial, including 55 patients with NAC 600mg bid for two months, there was significant reduction in hydrogen peroxide (H2O2) concentration in exhaled air condensate vs baseline after one month (p<0.007) and after two months (p=0.0001) of treatment (Significant reduction in the study group vs placebo at the end of first and second months) [20]. In a double blind, randomised, placebo-controlled trial in 120 patients with stable COPD treated with NAC 600mg B.I.D. (placebo controlled) for 1 year, the frequency of exacerbations was significantly reduced by 44% in the NAC group as compared to the placebo group (0.96 vs1.71 times/year, p = 0.019). The authors concluded that the reduction in the rate of COPD exacerbations might be related to the anti-oxidant and anti-inflammatory effects of NAC [21].
Stav D et al., reported that, NAC treatment in 24 patients in a dose of 600mg bid for six weeks had beneficial effect on physical performance probably due to improvement in air trapping [22]. BRONCUS (Bronchitis Randomised on NAC Cost-Utility Study), a randomised placebo controlled trial, 523 COPD patients were treated with 600mg of NAC(placebo controlled) in addition to the standard treatment. They were followed for three years. It was concluded that, NAC is ineffective at prevention of exacerbations in COPD patients [23].
A recent meta-analysis of data from eight trials (in 2214 patients) have shown that, NAC significantly reduced the number of exacerbations over the treatment period by about 50% (Odds Ratio (OR) 0.49, 95% CI, 0.32-0.74, p= 0.001). Overall analysis of this meta-analysis indicates that NAC, by reducing the rate of exacerbations may help in modifying the natural history of moderate to severe COPD [24].
Physical activity is recommended for all COPD patients [3]. Walking, an activity most people can perform, has been associated with nearly all the benefits of regular physical activity [25]. Garcia-Aymerich et al., reported 20 years follow-up of 2368 COPD patients, daily physical activity in the form of cycling or daily walk for two hours/week showed 30-40% reduction in the events of hospitalisation and mortality in a population based cohort. This was the first population based cohort study, showing that a relatively small amount of physical activity may have important beneficial effect on the course of COPD, as 30-40% reduction in hospitalisation indicates lesser numbers of exacerbations and improvement in quality of life, indirectly [26]. Maria T et al., reported in their study of walking as physical activity, improved exercise capacity, dyspnea and QoL in COPD patients [27].
Limitation
The sample size in this study was small (n=100). Non-availability of previous studies on combined effect of NAC and physical activity in literature was a limiting factor for sample size calculation. Daily physical activity record in this study was based on self reported charting by the patients. This may be modified by use of accelerometers (activity tracker), as objective method to record daily physical activity. SGRQ-C questionnaire is time consuming and requires complex calculations. SGRQ-C does not address psychological well being.
Conclusion
As our study results show statistically significant improvement in quality of life, with combination of 600mg of NAC per day with 20 minute daily walk, in addition to standard treatment in stable COPD patients can be considered as a part of management.
Registration: Trial was registered with Clinical Trial Registry of India Retrospectively-CTRI/2016/06/007024.
Paired t-test was applied.
Independent t – test was applied.
Results are based on nonempty rows and columns in each innermost sub table.
* The Chi-square statistics is significant at the 0.05 level.