The Dismal State of Pathology Laboratories in Dental Colleges – A Stitch in Time Can Save Nine!
Priyanka Kardam1, Shweta Rehani2, Sneha Sethi3
1 Senior Lecturer, Department of Oral Pathology and Microbiology, Sudha Rustagi College of Dental Sciences and Research, Faridabad, Haryana, India.
2 Reader, Department of Oral Pathology and Microbiology, Sudha Rustagi College of Dental Sciences and Research, Faridabad, Hayana, India.
3 Senior Lecturer, Department of Oral Pathology and Microbiology, Sudha Rustagi College of Dental Sciences and Research, Faridabad, Hayana, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Priyanka Kardam, Senior Lecturer, Department of Oral Pathology and Microbiology, Sudha Rustagi College of Dental Sciences and Research, Faridabad, Haryana, India.
E-mail: priyankakardam@gmail.com
Being a pathologist, we all are aware of the protocols which need to be followed in the histopathology laboratories. However, quite often due to our carelessness or busy schedules we tend to skip one or two steps in the protocol. These steps may appear to be insignificant at that time but later on they may take a toll on the diagnosis by creating an artefact. Here, we have presented a case of a similar artefact which tried to mask our diagnostic ability.
Artefact, Grossing, Myiasis, Worm
During routine histopathological evaluation of a slide of periapical granulation tissue, we encountered an uncommon structure (artefact) [Table/Fig-1a]. This structure was lying next to the granulation tissue without touching its boundaries and had taken up Hematoxylin and Eosin (H&E) stain. On doing literature search, the artefact in the tissue appeared to be a cross-section of a worm [Table/Fig-1b]. The size of artefact was so huge that even at scanner view both the artefact and granulation tissue could not be clicked in same focus.
a) Photomicrograph showing periapical granulation tissue (arrow). The worm artefact was nowhere touching the boundaries of this tissue (H&E staining, X10); b) Photomicrograph showing cross-section of worm (H&E staining, X10).
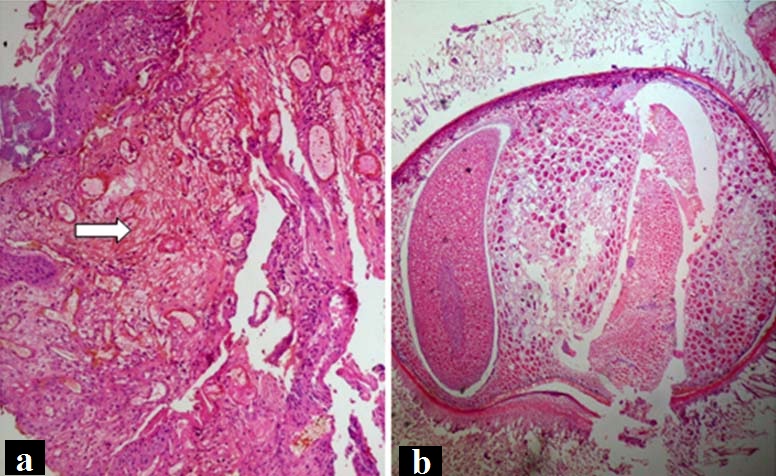
It was quite intriguing for us to find out how a worm tissue could get mixed up with an oral biopsy. The possible provisional diagnosis which came to our minds was myiasis (maggots). However, the possibility of the worm to be a case of myiasis was ruled out in this case as follows: Myiasis is caused by flies when they come in direct contact with tissue owing to open wounds, sores and ulcers. Since the present case was of a periapical lesion, there was no possibility of direct contact with flies. Secondly, maggots are known to erode surrounding tissue and release proteolytic enzymes that lead to tissue necrosis and destruction. Whereas, in our case, no necrotic tissue was obtained, as evident in the histopathologic image [Table/Fig-1a]. Thirdly, in case of myiasis, the maggot would have been entrapped within the tissue. However, as mentioned the artefact was lying away from the periapical tissue.
Although the occurrence of bizarre artefacts in biopsies has been previously reported, yet there are no reports in literature of appearance of a worm in an oral biopsy [1]. The possible reasons for this could be an inaccuracy in the standard operating protocol which needs to be followed from the time a tissue is excised by the surgeon till the time a histopathological slide reaches the pathologist for diagnosis [2].
Starting from the surgeon’s table, the sterilization cloth and instru-ments used can be a possible source of contamination. The next step as per the protocol is transferring the biopsied tissue into a bottle containing fixative. Ideally, a wide-neck sterile bottle is recommended to keep the tissue in fixative. However, the reality is that in most of the dental colleges and dental clinics, an empty local anaesthetic bottle is used for this purpose. This bottle is a risk for contamination as it may contain any foreign object or even microbial contamination which may appear as an artefact in the histopathologic slide and thus may obscure the diagnosis. In case this bottle is to be used we recommend that it should be meticulously cleaned and sterilized before use.
Moving ahead towards the grossing station, there are multiple risks for contamination of the tissue. The tissue comes in contact with grossing sheets which should be routinely washed and sterilized to avoid contamination. Secondly, the instruments used for grossing are the potent source for contamination and hence should be scrubbed/cleaned and sterilized routinely.
Next, during the processing of the tissue, it is important to maintain the concentration of the alcohols and other solutions used for dehydration. We also recommend that after every batch of tissues is dehydrated; the alcohols should be filtered using a filter paper so that any foreign object or piece of tissue if remaining in alcohol gets removed. Similarly, we also recommend that the wax used for impregnation and all the staining solutions should be filtered routinely to avoid incorporating any foreign-body in the final slide.
The purpose of this communication is to draw attention of the readers towards maintaining a standard, cleanliness and sterilization protocol during handling of the tissue. Though we all are aware of its importance, yet at times there is a glitch in the entire process owing to our carelessness. This creates a leeway for a foreign body to enter the tissue during fixation, grossing, tissue processing or impregnation and this may lead to an artefact which in turn may lead to a wrong diagnosis. If we maintain a proper protocol for keeping our laboratories clean and sterilized, there would be no chances of foreign bodies being incorporated as artefacts. This will save our time later while diagnosing the slide and avoiding erroneous diagnosis. For example in our case, myiasis could have been a misdiagnosis.
Recommendations
Unsterilized empty local anaesthetic bottles should not be used for storing biopsy specimens.
A clean and sterilized grossing sheet should be used for each biopsy tissue.
All the solutions used in tissue processing and staining should be filtered routinely using a filter paper.
[1]. Sonal G, Rashmi N, Jayadeva H, Ahmed Mujib B, Suture artefacts: Explored through polarising microscopeSultan Qaboos Univ Med J 2012 12:247-48. [Google Scholar]
[2]. Rastogi V, Puri N, Arora S, Kaur G, Yadav L, Sharma R, Artefacts: A diagnostic dilemma – A reviewJ Clin Diagn Res 2013 7:2408-13. [Google Scholar]