The pathogenesis of vitiligo is not yet understood. Multiple factors seem to play role in disease development [5]. Auto-immune destruction of melanocytes [6], neural mechanisms [7], oxidant/anti-oxidant imbalance [8] and intrinsic adhesion defect in melanocytes [9] all were suggested to explain the pathogenesis. Despite the interplay between melanocytes and neighboring keratinocytes to form epidermal melanin units, the role of keratinocytes in pathogenesis of vitiligo was not extensively investigated [10].
Some authors have suggested that keratinocytes have direct and indirect effects on melanocyte proliferation and differentiation through cell-cell adhesion and production of keratinocyte-derived growth factors. Thus, keratinocyte damage will be adversely reflected on melanocytes [11].
Electron microscopic examination of vitiliginous skin showed that basal and suprabasal keratinocytes degenerate, not only in depigmented but also in normally pigmented skin [12]. This cellular degeneration is due to either necrosis or apoptosis. Keratinocyte destruction by auto-antibodies has also been reported in vitiligo patients [13].
Cadherins are a family of Ca-dependent transmembrane proteins mediating specific homophilic cell–cell adhesion. E-Cadherin is the major adhesion molecule mediating melanocyte–keratinocyte interactions [14,15]. E-Cadherin dependent cell–cell adhesion is sensitive to environmental redox status and is highly dependent on Ca2+ level [16]. Both redox status and Ca2+ level are dysregulated in the epidermis of vitiligo patients, eventually implicating role of E-Cadherin in pathogenesis of vitiligo [17].
Aquaporins (AQPs) belong to a highly conserved group of membrane proteins called the Major Intrinsic Proteins (MIPs) which are present in all types of organisms and involved in the transport of water and small solutes such as glycerol, nitrate and urea [18]. There are 13 AQP isoforms in humans. They are divided into two groups based on their selective permeability; aquaporins (permeable only to water) and aquaglyceroporins (permeable to water and glycerol) [19]. AQP3 is the most widely expressed isoform in human skin [20]. AQP3 has been identified as a transmembrane protein, which has been localized to the basolateral membrane [21] and is delivered to post-golgi structures directly for the formation of cell-to-cell contacts, where it co-accumulates with E-cadherin. These findings suggest a connection between AQP3 and E-cadherin–catenin complex [22].
The current study aimed at investigating the keratinocyte cell-cell adhesion in perilesional vitiligo skin through the immunolocalization of AQP3 and E-cadherin.
Materials and Methods
Studied Population
This case-control study was carried out on 65 subjects which included 40 patients with vitiligo and 25 age and sex- matched normal subjects as a control group. Patients were selected from the dermatology outpatient clinic, Menoufiya University Hospital during the period between February 2012 to April 2013. Control subjects were selected from persons attending Plastic Surgery Department.
All studied patients were subjected to complete history taking, general and dermatological examination. Clinical data describing patients’ demographics (age and gender) as well as the clinical variables {site(s) affected, disease duration, duration of biopsoed lesion, presence of leukotrichia, presence of spontaneous pigmentation, disease course and family history} were all documented. Vitiligo was clinically classified according to Taieb A and Picardo M [23]. Selected cases were either newly diagnosed without history of previous treatment or old cases with completely depigmented lesions.
Assessment of disease activity was done according to Vitiligo Disease Activity (VIDA) score [24].
Exclusion Criteria
All subjects with dermatologic diseases other than vitiligo were excluded. Patients with systemic disorders were excluded. Patients receiving topical and/or systemic retinoid therapy, calcium and/or vitamin D supplements were also excluded.
Ethics
Written consent form approved by the Local Ethical Research Committee at Menoufiya Faculty of Medicine was obtained from every participant prior to the study initiation. This was in accordance with helsinky declaration in 1975 (revised in 2000).
Biopsies
From each patient, 5-mm punch biopsy was obtained, using 2% lignocaine local anaesthesia. Biopsies were obtained from perilesional skin which is 1-5mm peripheral to marginal area [25]. Biopsies from cases and control subjects were site-matched. All specimens were fixed in 10% neutral-buffered formalin and subjected to routine tissue processing that ended with paraffin-embedded blocks ready for sectioning at the Pathology Department, Faculty of Medicine, Menoufiya University.
Immunohistochemical Staining
Four-μm-thick sections were cut from the paraffin-embedded blocks with subsequent steps of deparaffinization and rehydration in xylene and graded series of alcohol, respectively. Antigen retrieval was performed by boiling in 10ml citrate buffer (pH 6.0) for 20 min, followed by cooling at room temperature. After cooling, the slides were incubated overnight at room temperature with:
- Mouse monoclonal antibody raised against E-cadherin. It was received as 7ml ready to use (Lab Vision, CA Cat. #MS-9470-R7).
- Rabbit polyclonal antibody raised against AQP3 {Abcam (ab125219)}. The optimal dilution was 1:500 by using phosphate buffered solution.
Detection of immunoreactivity was carried out using the ultravision detection system, ready-to-use anti-polyvalent horseradish peroxidase/diaminobenzidine (LabVision). Finally, the reaction was visualized by an appropriate substrate/chromogen (diaminobenzidine) reagent. Counter stain was carried out using Mayer’s haematoxylin.
Interpretation of Immunostaining
For E-cadherin: A brown cytoplasmic, membranous or membrano-cytoplasmic stain was considered positive in perilesional skin and control specimens [26].
For AQP3: A brown cytoplasmic, membranous or membrano-cytoplasmic stain was considered positive in perilesional skin and control specimens [27].
For both antibodies, the following items were evaluated in control and perilesional skin:
A-Inter-follicular and follicular epidermis were assessed for the following:
1- Expression: Positive or negative.
2- Intensity of the stain: Mild (+), moderate (++) or strong (+++).
3- Expression percentage: percentage of the positive cell was assessed at x200 magnification field [28].
4- Histo-score (H- score): H-score was calculated to all positive specimens according to the following equation:
H-score = 1 x % of mildly stained cells + 2 x % moderately stained cells + 3 x % of strongly stained cells [29].
5- Distribution: Patchy (irregular or not uniform) or Diffuse (uniform).
6- Cellular pattern: Cytoplasmic, Membranous or Membrano-cytoplasmic.
7- Localization: Basal layer or basal and suprabasal layers.
B-Dermis (adnexa, endothelial cells, fibroblasts and inflammatory cells) was assessed for Expression: Positive or negative.
Statistical Analysis
Results were collected, tabulated, and statistically analysed using an IBM personal computer and the statistical package SPSS version 11. Fisher-exact and Chi-square tests (χ2) were used to study the association between two qualitative variables. Mann-Whitney test (non-parametric test) was used for comparison between quantitative variables not normally distributed. Spearman’s correlation coefficient (r) was used to assess correlation between two quantitative variables. The p≤0.05 was considered significant.
Results
Studied Population
Clinical data of studied cases are summarized in [Table/Fig-1].
Clinical data of the studied cases.
| Study GroupN= 40 |
|---|
| Disease Duration (in months) |
| X±SD | 10.50±10.29 |
| Range | 1 – 60 |
| Duration of biopsied lesion (in months) |
| X±SD | 3.05±2.53 |
| Range | 0.25 – 10 |
| N | % |
| Family history |
| Positive | 13 | 32.5 |
| Negative | 27 | 67.5 |
| Course |
| Stationary | 19 | 47.5 |
| Progressive | 21 | 52.5 |
| Site |
| Face and scalp | 9 | 22.5 |
| Trunk | 10 | 25.0 |
| Extremities | 9 | 22.5 |
| Whole body | 7 | 17.5 |
| Scattered | 5 | 12.5 |
| Spontaneous pigmentation |
| Yes | 5 | 12.5 |
| No | 35 | 87.5 |
| Leucotrichia |
| Yes | 15 | 37.5 |
| No | 25 | 62.5 |
| Type of vitiligo |
| Focal | 10 | 25.0 |
| Acrofacial | 8 | 20.0 |
| Universal | 7 | 17.5 |
| Generalized | 8 | 20.0 |
| Segmental | 7 | 17.5 |
| Type of vitiligo |
| Non segmental | 33 | 82.5 |
| Segmental | 7 | 17.5 |
| VIDA Score |
| X±SD | 1.20±1.32 |
| Range | 1 – 4 |
X: Mean; SD: Standard deviation; VIDA: Vitiligo Disease Activity
Immunohistochemical expression of AQP3 in studied groups
1- Normal skin: Epidermal expression was positive in all examined sections with membranous and membrano-cytoplasmic patterns and diffuses distribution. Dermal expression was positive in all examined sections in monocytes, endothelial cells, sweat and sebaceous glands. Hair follicle expression was positive in 40% of examined sections with moderate and strong intensity. Detailed AQP3 expression in control skin is shown in [Table/Fig-2,3].
Comparison of AQP3 expression between perilesional and control skin.
| Variable | Perilesional skinN = 40 | Control skinN = 25 | Test ofsignificance | p-value |
|---|
| Epidermis | N | % | N | % |
|---|
| Expression |
| Positive | 40 | 100 | 25 | 100 | FE | 0.52 |
| Negative | 0 | 0.0 | 0 | 0.0 | 1.29 |
| Pattern |
| Cytoplasmic | 1 | 2.5 | 0 | 0.0 | χ2 | 0.001* |
| Membranous | 18 | 45.0 | 23 | 92.0 | 14.62 |
| Membranocytoplasmic | 21 | 52.5 | 2 | 8.0 | |
| Intensity |
| Mild | 4 | 10.0 | 0 | 0 | χ2 | <0.001* |
| Moderate | 29 | 72.5 | 7 | 28.0 | 19.88 |
| Strong | 7 | 17.5 | 18 | 72.0 | |
| Distribution |
| Patchy | 19 | 47.5 | 0 | 0 | χ2 | <0.001* |
| Diffuse | 21 | 52.5 | 25 | 100 | 16.78 | NA |
| Localization |
| Basal | 0 | 0 | 0 | 0 | χ2 | <0.001* |
| Basal and suprabasal | 40 | 100 | 25 | 100 | ---- |
| Percent |
| X±SD | 69.75±14.05 | 94.20±6.24 | U | <0.001* |
| Range | 40 – 90 | 40 – 90 | 80 – 100 | 80 – 100 | 6.21 |
| H-score |
| X±SD | 124.25±33.73 | 261.0±52.08 | U | <0.001* |
| Range | 60 – 190 | 60 – 190 | 160 – 300 | 160 – 300 | 6.46 |
| Dermis | N | % | N | % | | |
| Expression |
| Positive | 40 | 100 | 25 | 100 | χ2 | NA |
| Negative | 0 | 0.0 | 0 | 0.0 | ---- |
| Hair follicle | N | % | N | % | | |
| Expression |
| Positive | 31 | 77.5 | 10 | 40 | χ2 | 0.002* |
| Negative | 9 | 22.5 | 15 | 60 | 9.29 |
| Intensity |
| Mild | 19 | 61.3 | 0 | 0 | χ2 | <0.001* |
| Moderate | 12 | 38.7 | 3 | 30.0 | 27.99 |
| Strong | 0 | 0.0 | 7 | 70.0 | |
| Percent |
| X±SD | 42.42±16.27 | 93.5±7.09 | U | <0.001* |
| Range | 20 – 70 | 80 – 100 | 4.74 |
| H-score |
| X±SD | 60.97±30.15 | 263.5±52.18 | U | <0.001* |
| Range | 20 – 140 | 160 – 300 | 4.73 |
X: Mean; SD: Standard deviation; NA: Not Applicable; *: Significant; FE: Fisher-exact test; U: Mann Whitney test; χ2: Chi-square test
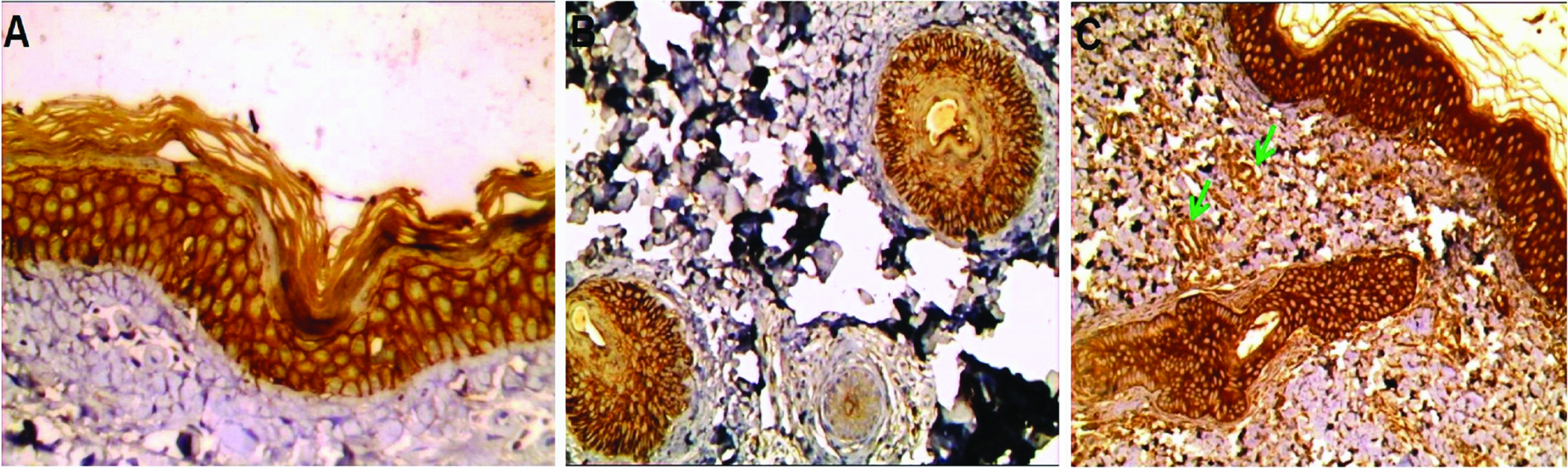
AQP3 expression in normal skin: a) Strong membranous and membrano-cytoplasmic diffuse expression in basal and suprabasal layers of inter-follicular epidermis (immunoperoxidase 40X); b) Strong membranoctoplasmic expression in follicular epidermis (immunoperoxidase, 20X); c) Strong membranous and membranocytoplasmic expression in follicular and inter-follicular epidermis with positive endothelial immunoreactivity (green arrows) (immunoperoxidase, 20X)
2- Perilesional vitiligo skin: Epidermal expression was positive in all examined cases with cytoplasmic, membranous and membranocytoplasmic patterns. Distribution was patchy or diffuse. Localization was basal and suprabasal in all examined cases. Dermal expression was positive in all examined cases in monocytes, sweat and sebaceous glands. Hair follicle expression was positive in 77.5% of cases with mild or moderate intensity. Detailed AQP3 expression in perilesional skin is shown in [Table/Fig-2,4].
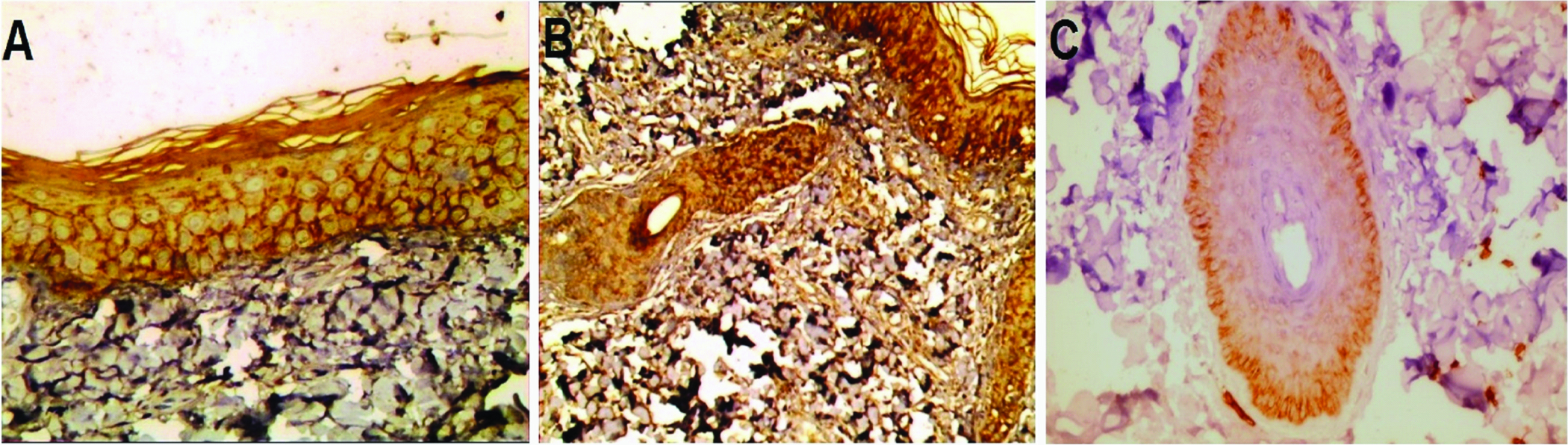
AQP3 expression in perilesional vitiligo: a) Moderate membranous and membrano-cytoplasmic diffuse expression in basal and suprabasal layers of interfollicular epidermis (immunoperoxidase 40X); b) Mild with foci of moderate cytoplasmic expression in follicular and inter-follicular epidermis (immunoperoxidase 20X); c) Mild cytoplasmic expresion in follicular epidermis that occupied suprabasal layer (immunoperoxidase 40X).
Comparison between perilesional and control skin regarding AQP3 expression
In inter-follicular epidermis: Membranous pattern (p= 0.001), strong intensity, diffuse distribution, higher percent and higher H-score (p<0.001 for all) were significantly associated with control skin compared with perilesional skin [Table/Fig-2].
In follicular epidermis: Negative hair follicle expression (p= 0.002), strong intensity, higher percent and higher H-score (p<0.001 for all) were significantly associated with control skin compared with perilesional skin [Table/Fig-2].
Immunohistochemical expression of E- Cadherin in studied groups:
1- Normal skin: Epidermal expression was positive in all examined sections with membranous and membrano-cytoplasmic patterns and diffuses distribution. Dermal expression was positive in all examined cases in endothelial cells and monocytes. Hair follicle expression was positive in 40% of examined sections with moderate or strong intensity and diffuses distribution. Detailed E-cadherin expression in control skin is shown in [Table/Fig-5,6].
Comparison between e-Cadherin expression in perilesional and control skin and control skin.
| Variable | Perilesional skinN = 40 | Control skin N = 25 | Test of significance | p-value |
|---|
| Epidermis | N | % | N | % |
|---|
| Expression |
| Positive | 38 | 95.0 | 25 | 100 | FE | 0.52 |
| Negative | 2 | 5.0 | 0 | 0.0 | 1.29 |
| Pattern |
| Membranous | 34 | 89.5 | 13 | 52.0 | χ2 | 0.001* |
| Membranocytoplasmic | 4 | 10.5 | 12 | 48.0 | 11.17 |
| Intensity |
| Mild | 17 | 44.7 | 0 | 0 | χ2 | <0.001* |
| Moderate | 18 | 47.4 | 22 | 88.0 | 15.37 |
| Strong | 3 | 7.9 | 3 | 12.0 | |
| Distribution |
| Patchy | 0 | 0 | 0 | 0 | χ2 | NA |
| Diffuse | 38 | 100 | 25 | 100 | ---- |
| Cellular localization |
| Basal | 0 | 0.0 | 0 | 0 | χ2 | NA |
| Suprabasal | 0 | 0.0 | 0 | 0.0 | ---- |
| Basal and supra basal | 38 | 100 | 25 | 100 | |
| Percent |
| X±SD | 53.68±22.95 | 83.0±10.89 | U | <0.001* |
| Range | 10 – 90 | 10 – 90 | 50 – 95 | 50 – 95 | 5.35 |
| H- score |
| X±SD | 80.78±42.20 | 177.2±51.05 | U | <0.001* |
| Range | 10 – 150 | 10 – 150 | 40 – 270 | 40 – 270 | 5.86 |
| Dermis | N | % | N | % | | |
| Expression |
| Positive | 38 | 95.0 | 25 | 100 | FE | 0.52 |
| Negative | 2 | 5.0 | 0 | 0.0 | 1.29 |
| Hair follicle | N | % | N | % | | |
| Expression |
| Positive | 23 | 57.2 | 10 | 40.0 | χ2 | 0.17 |
| Negative | 17 | 42.5 | 15 | 60.0 | 1.89 |
| Intensity |
| Mild | 15 | 62.5 | 0 | 0 | χ2 | <0.001* |
| Moderate | 8 | 37.5 | 6 | 60.0 | 16.77 |
| Strong | 0 | 0.0 | 4 | 40.0 | |
| Percent |
| X±SD | 34.78±22.33 | 85.0±7.45 | U | <0.001* |
| Range | 10 – 80 | 70 – 90 | 4.40 |
| H-score |
| X±SD | 49.56±37.32 | 206.5±53.02 | U | <0.001* |
| Range | 10 – 120 | 140 – 270 | 4.51 |
X: Mean; SD: Standard deviation; NA: Not Applicable; *: Significant; FE: Fisher-exact test; U: Mann Whitney test; χ2: Chi-square test
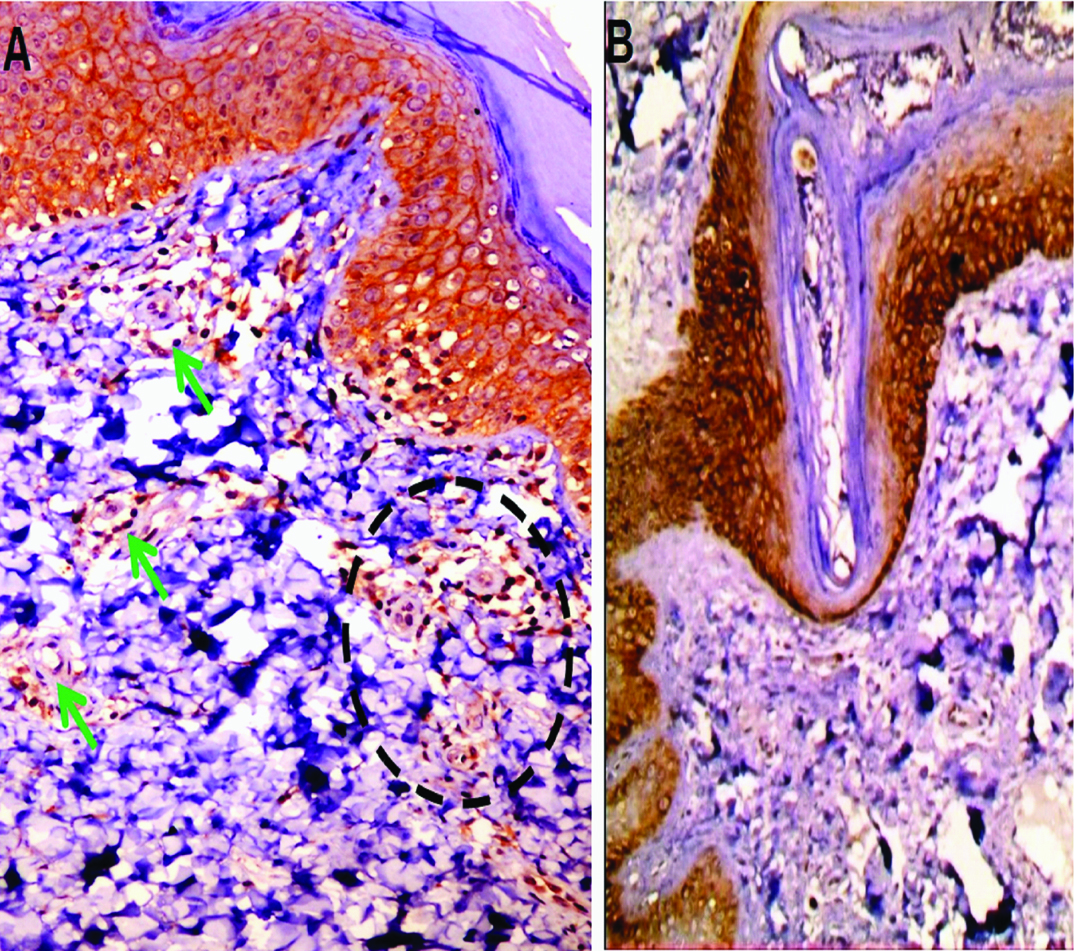
E-cadherin expression in normal skin: a) strong membranous and membrano-cytoplasmic diffuse expression in basal and suprabasal layers of inter-follicular epidermis. Positive immunoreactivity was detected in monocytes (dashed circle) and endothelial cells (green arrows). (immunoperoxidase, 40X); b) Strong membranous and membranocytoplasmic diffuse expression in follicular epidermis (immunoperoxidase 20X)
2- Perilesional vitiligo skin: Epidermal expression was positive in 95% of cases with membranous and membranocytoplasmic patterns and diffuse distribution. Dermal expression was positive in 95% of cases in endothelial cells, inflammatory cells, sweat and sebaceous glands. Hair follicle expression was positive in 57.2% of cases with mild or moderate intensity. Detailed E-cadherin expression in perilesional skin is shown in [Table/Fig-5,7].
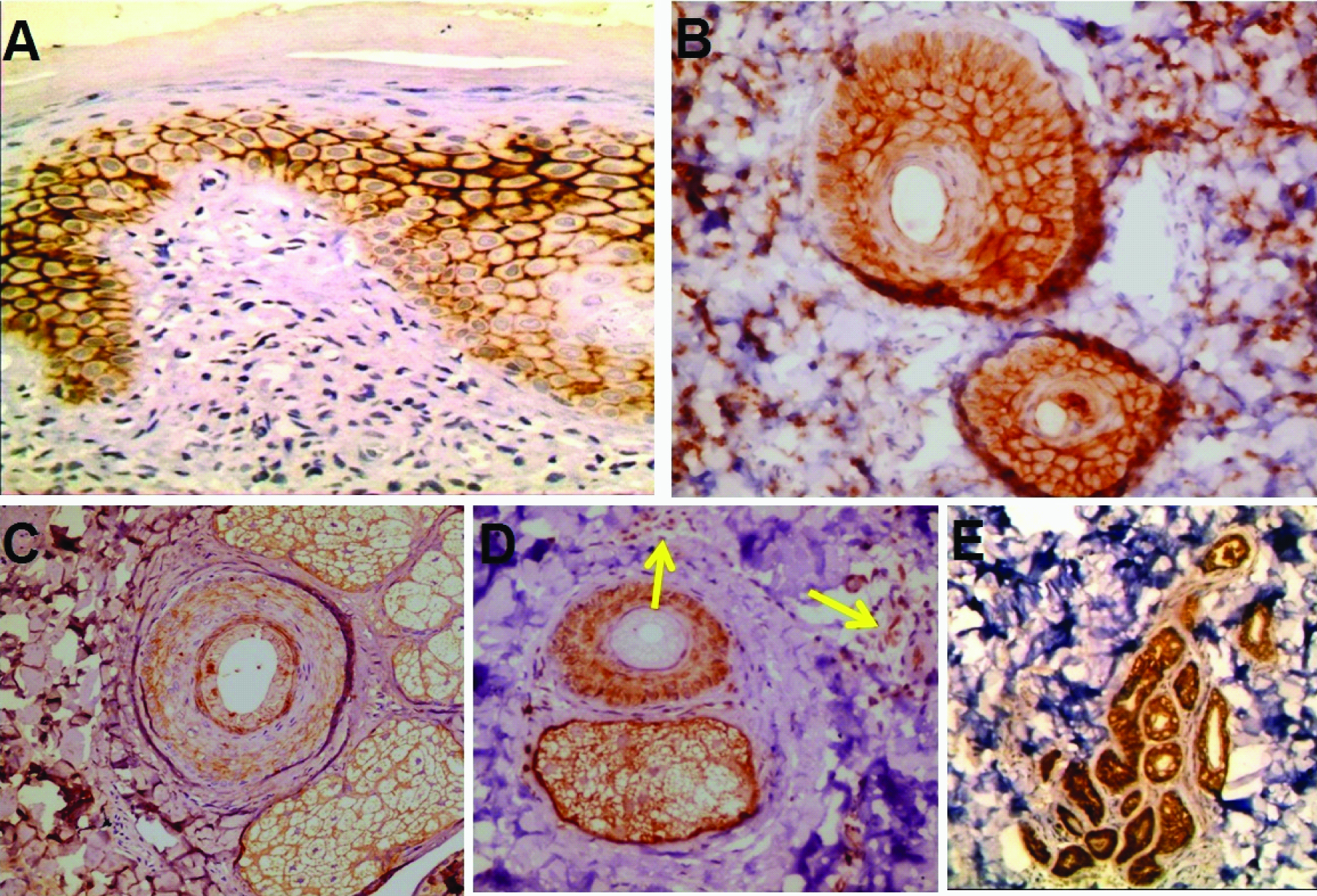
E- cadherin expression in perilesional vitiligo: a) Mild with foci of strong membranous patchy expression of inter-follicular epidermis (immunoperoxidase, 40X); b) Mild expression in follicuar epidermis (immunoperoxidase, 40X); c) mild membranous expression in follicular epidermis and sebaceous glands (immunoperoxidase, 40X); d) Mild membranous and membranocytoplasmic expression in follicular epidermis and sebaceous glands with positive immunoreactivity in inflammatory cells (arrows) (immunoperoxidase, 20X); e) Positive membranous expression in sweat glands (immunoperoxidase, 20X).
Comparison between e-Cadherin expression in perilesional and control skin
In inter-follicular Epidermis: membranocytoplasmic pattern (p< 0.001), moderate intensity, higher percent and higher H- score (p<0.001 for all) were significantly associated with control skin compared with perilesional skin [Table/Fig-5].
In follicullar epidermis: Moderate intensity, higher percent and higher H-score (p <0.001 for all) were significantly associated with control skin compared with perilesional skin [Table/Fig-5].
Relationship between E-cadherin and AQP3 H-scores and percent of expression and clinical data of selected cases: No significant association was found between E-cadherin and AQP3 H-scores and percent of expression and clinical data of selected cases either in follicular or inter-follicular epidermis.
No significant correlation was detected between E-cadherin and AQP3 H-scores and percent of expression and age of cases, disease duration or VIDA score either in follicular or inter-follicular epidermis.
Discussion
Phosphatidylinositol 3 kinase (PI3K)/AKT pathway, which is dependant on E-cadherin mediated cell adhesion and tyrosine phosphorylation, is essential for survival of differentiating cells [30]. AQP3 has been reported to directly target E-cadherin-mediated cell-to-cell adhesion [22], suggesting that AQP3 may have a role in the PI3K activation with E-cadherin [31].
The downregulation of AQP3, in cultured normal human keratinocytes, resulted in significantly decreased phosphorylation of PI3K-p85α and decreased expression of the E-cadherin–catenins. Moreover, AQP3 knockdown also reduced binding of E-cadherin and β-catenin to the same quantities of PI3K-p85α [31].
Therefore, AQP3 downregulation will adversely affect its downstream signalling molecules such as PI3K, E-cadherin, and catenins which are involved in the regulation of keratinocyte survival. Reduced survival of keratinocytes might lead to passive melanocyte death by loss of cell-cell contact and by reduction of keratinocyte-derived growth factors [32].
Epidermal melanocytes form functional and structural units with neighbouring keratinocytes. Structural abnormalities in keratinocytes have been considered to affect melanocyte growth and survival in vitro [33].
Furthermore, loss of adhesion between keratinocytes and melanocytes leads to detachment of melanocytes from basal to suprabasal layer of epidermis where they seem to be more susceptible to apoptosis [34].
The present study showed AQP3 and E-cadherin downregulation in perilesional skin compared with normal skin in follicular and inter-follicular epidermis. AQP3 was previously reported to be downregulated in lesional vitiligo skin [35]. It was not addressed before in perilesional skin. Wagner RY et al., demonstrated absence and discontinuous distribution of E-cadherin in perilesional normal-appearing skin leading to altered cell–cell adhesion [34]. Authors added that, this downregulation was associated with oxidative stress in affected cells manifested by increased Reactive Oxygen Species (ROS) production, increased lipid peroxidation and melanocyte detachment from basement membrane with H2O2 application in culture.
This observation underscores previous studies reporting melanocyte detachment following mechanical trauma due to primary intrinsic adhesion defect [9] and with decreased melanocyte number in basal cell layer of re-constructed skin from healthy subjects [36].
However, in mice with E-cadherin-deficient melanocytes, depigmentation occurs only after mechanical stress that is caused by repeated brushing of tail skin and in a model of reconstructed epidermis with normal melanocytes after exposure to H2O2 that leads to E-cadherin destabilization. In skin biopsies from patients with vitiligo, E-cadherin alterations are associated with lipoperoxidative damage. Therefore, persistent and widespread stress affecting the distribution of E-cadherin across melanocyte membranes in patients with vitiligo, before the appearance of clinical lesions, cause functional impairment, arguing for primary defects in melanocytes [37].
From these observations in perilesional vitiligo skin, we can postulate that therapeutic options for vitiligo has to be extended to pigmented skin which may help to prevent early events in “silent” vitiligo melanocytes and prevent the spread of the disease.
And now, a question arises; what is the precipitating factor that leads to AQP3 downregulation in perilesional vitiligo skin with all its subsequent events? Is it affected by oxidative stress, by mineral concentrations like Ca2? Or there are unknown controlling mechanisms? The answer requires more molecular investigation to be demystified.
Limitation
The limitation of this study was that a small number of cases was included. Further large-scaled studies are needed. Other investigatory techniques like Polymerase Chain Reaction (PCR) may be used to evaluate AQP3 and E-cadherin expression in perilesional vitiligo skin. Clinical trials using topical formulations containing AQP3 are needed to evaluate its therapeutic efficacy on vitiligo lesions and its ability to stop disease progression if applied to perilesional areas.
Conclusion
From the above mentioned results, we can suggest that there may be a primary event leading to AQP3 downregulation in normal skin before the appearance of vitiliginous lesions. This first step, is never observed in normal skin, even if trauma or chemical stressor could be present. So, a sort of priming factor could be operative in order to develop the vitiliginous patches. This first pathogenetic step is the initiating factor. Accordingly, the sequence of events may be as follows: AQP3 and its downstream molecules (P3IK, E-cadherin and catenins) downregulation by a primary unknown factor which is followed by defective keratinocyte adhesion and decreased relaease of keratinocyte-derived growth factors. Subsequently secondary pathogenic stimuli, notably physical trauma, oxidative stress or auto-antibodies, may lead to exfoliation of keratinocytes and pigmented cells. Melanocytes detach from the basal membrane toward the stratum corneum and exfoliate together with the surrounding keratinocytes. Further large-scaled research is mandatory to prove or deny current observation.