Bronchial asthma is a common chronic airway inflammatory disease prevalent across all age group. Current global prevalence of asthma is 1-18% as reported from different countries [1]. The disease heterogeneity characterized by variable symptoms and expiratory airflow limitations, along with complex underlying disease pathogenesis, attributes to highly variable ‘asthma phenotype’ [1].
Inhaled Corticosteroids (ICS) play a vital role in asthma management for the past 3 decades. Nonetheless, substantial inter-individual variability in the response to corticosteroids is being reported. ICSs alleviate clinical symptoms, improve pulmonary function, and reduce airway inflammation but higher doses and prolonged use of ICSs can have systemic effects [2]. The efficacy of various ICS with respect to specific delivery devices was studied through Randomized Control Trials (RCT) but there is no standardized method to compare their beneficial and adverse effects [2]. However, Pulmonary Function Tests (PFT) parameters such as percent change in FEV1 (Forced Expiratory Volume in 1 second) are used for assessing efficacy of ICSs over a period of treatment [3].
Chronic airway inflammation in asthma is characterized by a complex cascade of immunological events mediated by eosinophil recruitment and activation, which is yet to be explored and understood. Fractional exhaled Nitric Oxide (FeNO) is investigated as the surrogate marker of airway inflammation which is closely associated with bronchial eosinophilia. FeNO is currently probed to explain asthma molecular phenotype, assess severity of inflammation and predict corticosteroid responsiveness [4]. Association of FeNO with airway inflammation is supported by many studies reporting increased Nitric Oxide (NO) levels in subjects with asthma, decrease in levels on treatment with ICS and correlation with sputum eosinophil [5–9]. American Thoracic Society (ATS) guidelines recommend FeNO in asthma management and predicting ICS response [10].
A recent systematic review based on six different RCTs emphasize the importance of FeNO monitoring in asthma management especially in disease severity assessment which may be beneficial in controlling severe exacerbations [11]. In a global real life survey, FeNO was found to be significantly higher in patients with poorly controlled asthma, along with or without co-morbidities. Moreover, FeNO values were found to be increased in patients with the lowest corticosteroid dose [12]. In a recent study, FeNO values along with other immunological parameters such as Immunoglobin E (IgE) was found to be a good predictor of Asthma-COPD (Chronic Obstructive Pulmonary Disease) Overlap Syndrome (ACOS), in COPD population [13]. In a study to explore the possible lower airway abnormalities in Non-Allergic Rhinitis (NAR) patients without asthma, FeNO had shown significant correlation with positive airway hyper-responsiveness and allergic rhinitis [14]. Moreover, FeNO based grouping of asthma patients had provided independent classification of asthma severity in previous studies [15]. Till date, very few Indian studies are available reporting FeNO use in asthma diagnosis [16–19].
With this background, the present study aimed to determine the clinical utility of FeNO as a diagnostic biomarker of disease severity and ICS response in Tamil patients with persistent asthma.
Materials and Methods
The Institute Ethics Committee approval was obtained prior to the start of the study (Project No. JIP/IEC/SC/2013/3/411). The procedures followed were in accordance with the ethical standards stated in ICMR guidelines for human experimentations and with the Helsinki Declaration of 1975, revised in 2000. The present study was a prospective cohort undertaken in the OPDs of JIPMER hospital for a period of two years from November 2013 to October 2015. The sample size for the present study was calculated using PS software, considering minimum required difference in PFT parameter, FEV1, as the study population was sub-grouped based on FEV1 for both asthma severity and ICS response.
Both, male and female patients with diagnosed or symptomatically suspected asthma attending JIPMER hospital were approached for participation in the study and recruited based on the inclusion and exclusion criteria of the study. Written informed consent was obtained from each participant before recruitment. Inclusion criteria were those diagnosed with mild-to-moderate persistent asthma of Tamilian origin (residing in Tamilnadu or Pondicherry for the past three generations and with Tamil language as their mother tongue), aged 18-50 years of either gender who were treatment naive or without ICS treatment in last two months or more. Exclusion criteria were pregnant and lactating women, chronic smokers (i.e., >20 pack years; 1 Pack year =1 pack/day for 1 year), those under leukotriene antagonists, anti-IgE and other steroid based medications, with COPD reported through <12% change in FEV1, those with concurrent upper or lower respiratory tract infections and Tuberculosis positive.
Five milliliter of venous blood was collected from all the participants and treated with Ethylene diamine tetraacetic acid (EDTA) for laboratory investigations. Total blood percent eosinophil count, total blood percent neutrophil count and absolute eosinophil count were the haemotological parameters investigated as given in patient demographic details [Table/Fig-1].
Patient demographic details.
| Patient demographic details | |
|---|
| No. of patients recruited | 157 |
| Male : Female | 41:116 |
| Age, in yrs | 36.72 |
| No. of patients completed Follow-up period (8 weeks) | 102 |
| Male : Female | 15:87 |
| Age, in yrs – Mean (SD) | 37.1 (9.2) |
| Body Mass Index- Mean (SD) | 23.52 (4.7) |
| Smoking history (Ex:Current: Non-Smokers) | 06:07:89 |
| Alcohol consumption or tobacco use (Y/N) | 09/93 |
| Asthma History, in yrs- Mean (SD) | 5.24 (4.1) |
| Allergy History, n (%) |
| No Allergy | 55 |
| Dust (including Rhinitis) | 45 |
| Skin (including Dermatitis) | 02 |
| History of exacerbations/ ED visits/OCS use in the last 6 months | 13 |
| Hematological parameters- Mean (SD) |
| Total Blood Percent Eosinophil | 6.67 (5.86) |
| Total Blood Percent Neutrophil | 60.57 (11.73) |
| Absolute Eosinophil Count | 0.47 (0.73) |
Data expressed as Mean (SD); ED-Emergency department; OCS-Oral Corticosteroids
Clinical Investigations
Pulmonary Function Tests (PFT): As a preliminary step, suspected and known patients were screened using PFT which was performed using portable spirometry (Medikro® Spirostar). Persistent asthma diagnosis in the case of new patients was primarily based on reversibility test (>12% and 200ml change in FEV1) after salbutamol nebulization and secondarily on symptomatic history and clinical examinations. PFT parameters such as Forced Vital Capacity (FVC) in liters, FEV1 in liters, FEV1/FVC ratio, Forced Expiratory Flow 25%-75% (FEF 25-75%) and Peak Expiratory Flow Rate (PEFR) in liters per minute were measured at baseline (0 week) and follow-up (End of 8th week) visits, as per standard procedures. Three consecutive measurements were taken for each patient in each visit and the best of above three measurements in each visit was reported to be clinically meaningful for further analysis. Based on PFT parameters, patients were classified as mild or moderate persistent asthmatics, as per ATS guidelines [20].
Fractional exhaled Nitric Oxide (FeNO): FeNO level was measured using a chemiluminescence based exhaled breath analyser (Bedfont® NOBreath), at baseline (0 week) and follow-up (End of 8th week) visits. Precautionary steps to minimize confounding variables (no food intake or smoking before 1 hour, thorough mouthwash) were incorporated during the measurements, as per ATS guidelines [11]. Three consecutive measurements were taken for each patient in each visit and the average of the three was considered for further analysis.
ICS therapy, follow-up and compliance
Beclomethasone dipropionate (Beclate®- 200 mcg b.d) was prescribed as a standard treatment regimen along with symptom reliever medication, salbutamol (Asthalin®) and Rotahaler® device for inhalation, to all patients. They were followed up for a period of eight weeks. The technique to use Rotahaler® device with rotacaps was instructed with care to each participant and its proper usage by the patient was also checked at baseline. Patient compliance to the drug was determined through phone calls, pill count and prescription checking, in regular intervals and during their scheduled visits to the hospital.
Based on significant increase in percentage change in FEV1 during treatment, good and poor ICS responders were classified. The percentage change in FEV1 was defined as, i.e., ΔFEV1 % PREDICTED = % PRED FEV1 Pre-Broncho Dilation (Pre-BD) after treatment - % PRED FEV1 (Pre-BD) before treatment / % PRED FEV1 (Pre-BD) before treatment * 100. More than or equal to 8% change in percent predicted FEV1 (Pre-Broncho dilation) (Pre and post ICS treatment; 8 weeks) is considered as the cut-off for good response definition in the present study [3].
Statistical Analysis
SPSS version 19.0 was used for all statistical analysis. Data was expressed in mean and percentages. Significant difference in Pre-Post-Bronchodilation (BD) and baseline follow-up PFT as well as FeNO parameters were analysed using paired t-test. One-way Analysis of Variance (ANOVA) or unpaired t-test was used to test the significant difference in mean decrease of FeNO levels between groups (mild vs moderate vs moderately severe persistent asthmatics; ICS good vs poor responders). Fischer-exact test was used to test the significant correlation of FeNO levels with severity and also with ICS response. Predictive association of FeNO with disease severity and ICS response was analysed using Receiver Operating Characteristics (ROC) curve. The p<0.05 was considered statistically significant.
Results
Out of 215 patients screened through PFT for diagnosis of mild-to-moderate persistent asthma, 157 met the inclusion criteria and were recruited. Patient demographic details are summarized in the [Table/Fig-1]. A total of 102 patients completed the follow-up period of eight weeks.
Pulmonary Function Tests (PFT): Baseline PFT parameters considering both pre and post bronchodilation test are given in the [Table/Fig-2]. PFT measurements of patients showing more than or equal to 80% adherence to the drug were considered for follow-up analysis. In overall study, population (n=102) and ICS responders group (n=69), except for FEV1/FVC ratio, all other PFT parameters showed significant increase with respect to follow-up measurements than in baseline. In ICS poor responders group (n=33), no significant difference was observed [Table/Fig-3]. Moreover, a trend of overall decrease in mean values was observed in follow-up measurements of ICS poor responders, though not a statistically significant decrease.
Baseline pulmonary function test parameters* (n=102).
| Parameters | Pre-BD value (ml) | Pre-BD %pred | Post-BD value (ml) | Post-BD %pred | Mean value change (95%CI) | Mean % change (95%CI) |
|---|
| FVC (l) | 1.94 (0.7) | 65.0 (18.5) | 2.2 (0.8) | 73.9 (17.9) | 0.26 (0.05- 0.48) | 8.90 (3.87-13.93) |
| FEV1 (l) | 1.48 (0.6) | 58.7 (16.5) | 1.84 (0.7) | 71.7 (19.1) | 0.36 (0.18-0.54) | 13.0 (8.07-17.93) |
| FEV1/FVC | 78.5 (13.0) | 96.08 (16.1) | 82.92 (11.0) | 101.4 (13.4) | 4.42 (1.10-7.75) | 5.32 (1.23-9.41) |
| PEFR (l/s) | 3.37 (1.3) | 52.42 (18.4) | 4.1 (1.5) | 63.9 (20.9) | 0.73 (0.34-1.12) | 11.48 (6.04-16.92) |
| FEF25-75% | 1.53 (0.9) | 42.72 (22.82) | 2.5 (1.2) | 67.1 (33.0) | 0.97 (0.68-1.26) | 24.38 (16.55-2.21) |
FN: Data expressed as Mean (SD). Data analysed using paired t-test. BD-Bronchodilation with Salbutamol nebulization; FVC - Forced Vital Capacity (l); FEV1 - Forced Expiratory Volume in 1.0 second (l); PEFR- Peak Expiratory Flow Rate (l/s); FEF 25-75%- Forced Expiratory Flow Rate at 25-75%.*p<0.05.
Difference in pulmonary function test parameters.
| Parameter | Follow-up completed population (n=102) | ICS Good responders (n=69) | ICS Poor responders (n=33) |
|---|
| Pre-ICS | Post-ICS | Absolute Diff. (95% CI) | Percentage Diff. (95% CI) | Pre-ICS | Post-ICS | Absolute Diff. (95% CI) | Percentage Diff. (95% CI) | Pre-ICS | Post-ICS | Absolute Diff. (95% CI) | Percentage Diff. (95% CI) |
|---|
| FVC (l) | 1.94 (0.7) | 2.31 (0.8) | 0.38* (0.27-0.49) | 11.97* (9.05-14.89) | 1.87 (0.7) | 2.37 (0.8) | 0.51* (0.37-0.64) | 16.4* (13.7-19.1) | 2.22 (0.8) | 2.22 (0.7) | 0.00 (-0.19-0.19) | -0.26 (-6.6-6.1) |
| FEV1 (l) | 1.48 (0.6) | 1.82 (0.7) | 0.35* (0.25-0.45) | 11.97* (9.05-14.89) | 1.36 (0.54) | 1.92 (0.7) | 0.56* (0.44-0.7) | 17.0* (14.9-19.1) | 1.78 (0.6) | 1.70 (0.6) | -0.07 (-0.18-0.03) | -2.41 (-5.89-1.07) |
| FEV1/ FVC | 78.5 (13.0) | 78.42 (11.9) | -0.05 (-2.72-2.62) | -0.88 (-3.32-3.15) | 78.7 (13.2) | 79.99 (10.9) | 1.3 (-1.35-3.91) | 1.52 (-1.7-4.73) | 77.8 (12.7) | 74.04 (13.5) | -3.78 (-10.95-3.39) | -4.56 (-13.14-4.03) |
| PEFR (l/s) | 3.37 (1.3) | 4.06 (1.7) | 0.69* (0.43-0.96) | 11.2* (7.51-14.88) | 3.25 (1.3) | 4.15 (1.7) | 0.89* (0.56-1.22) | 15.1* (10.7-19.5) | 3.78 (1.5) | 3.78 (1.5) | 0.00 (-0.36-0.36) | 0.37 (-4.69-5.43) |
| FEF 25-75% | 1.53 (0.9) | 1.89 (1.1) | 0.36* (0.17-0.55) | 8.69* (3.96-13.42) | 1.48 (0.9) | 2.01 (1.1) | 0.53* (0.29-0.77) | 13.3* (7.73-18.87) | 1.70 (0.9) | 1.52 (0.9) | -0.19 (-0.46-0.08) | -3.78 (-11.30-3.75) |
FN: Data expressed as Mean (SD). Data analysed using paired t-test. p<0.05 considered as significant. ICS-Inhaled Corticosteroids; CI-Confidence Interval; FVC - Forced Vital Capacity (l); FEV1 - Forced Expiratory Volume in 1.0 second (l); PEFR- Peak Expiratory Flow Rate (l/s); FEF 25-75%- Forced Expiratory Flow 25-75%.*p<0.0001
Fractional exhaled Nitric Oxide (FeNO): The observed baseline and follow-up FeNO values are summarized in [Table/Fig-4], with respect to disease severity and ICS response (mild vs moderate vs moderately severe persistent asthmatics; ICS good vs poor responders). Difference in baseline and follow-up FeNO levels was found to be significant (p<0.0001) in overall population (n=102), moderate persistent (n=22), moderately severe persistent (n=54), in case of baseline FeNO levels more than 50 ppb (n=85) and in ICS good responders (n=69). Difference in baseline and follow-up FeNO levels was found to be comparatively less significant (p=0.001) among mild persistent asthmatics (n=26) and ICS poor responders (n=33) compared to the above. No significant difference in baseline and follow-up FeNO levels was observed in the cases with baseline FeNO levels less than 50 ppb (n=17). This could be due to less sample size in the group.
No significant difference in mean decrease of FeNO levels was observed between mild, moderate and moderately severe asthmatics groups (p=0.272). Moreover, the mean baseline FeNO levels were found to be similar in all the above asthmatics groups. This was found to be the same for ICS responder groups with similar mean baseline FeNO levels and no significant difference in mean decrease of FeNO levels was observed between ICS good and poor responders groups (p=0.18). Moreover, as per ATS guidelines 2011, patients showing at least 20% decrease in FeNO levels on ICS treatment are more likely to respond to the drug than others. In the present study, 20% or more decrease in mean FeNO values was not achieved in both the ICS responders group defined by PFT parameters. Though the FeNO reduction on ICS therapy was found to be statistically significant, clinically significant difference was not observed between ICS good and poor responders groups.
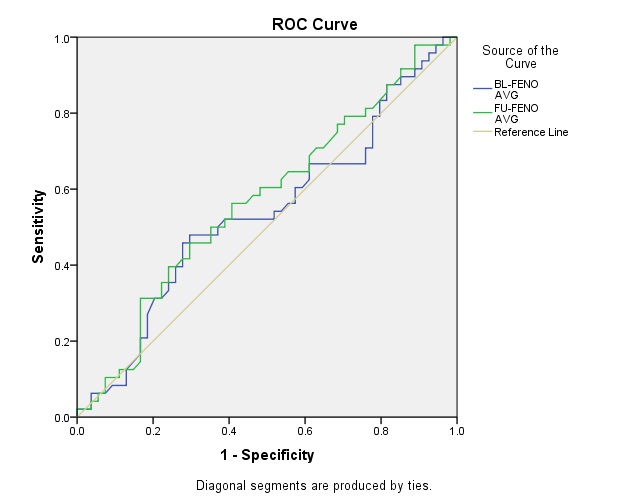
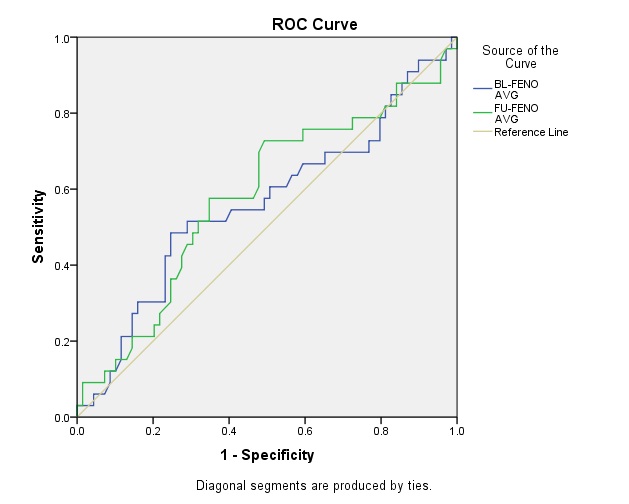
Receiver Operating Characteristics (ROC) curve analysis showed the AUC as 53.7% and 57.0% for predictive association of baseline and follow-up FeNO levels with asthma severity respectively [Table/Fig-5]. Similarly, the AUC was found to be 56.1% and 58.0% for predictive association of baseline and follow-up FeNO levels with ICS response [Table/Fig-6]. Predictive values of FeNO for asthma severity and ICS response at various cut-off ranges are given in [Table/Fig-7].
Pre- & Post- ICS treatment FeNO levels.
| Population | N | Pre-treatment FeNO (ppb) | Post-treatmentFeNO (ppb) | Mean Diff. (95% CI) |
|---|
| Overall | 102 | 97.80 (40.08) | 80.18 (31.57) | 17.62$ (13.43-21.81) |
| Mild | 26 | 103.03 (34.08) | 90.15 (27.36) | 12.87# (5.81-19.93) |
| Moderate | 22 | 91.38 (37.60) | 75.74 (31.98) | 15.64$ (9.29-21.98) |
| Moderately Severe | 54 | 97.90 (43.84) | 77.18 (32.79) | 20.72$ (13.93-27.52) |
| FeNO<50ppb | 17 | 35.88 (11.46) | 32.92 (20.29) | 2.96 (-4.57-10.49) |
| FeNO>50ppb | 85 | 110.18 (31.23) | 89.63 (24.04) | 20.56$ (15.96-25.15) |
| ICS-Good Responders | 69 | 97.12 (42.04) | 77.90 (31.12) | 19.22$ (14.1-24.4) |
| ICS-Poor Responders | 33 | 99.68 (34.71) | 86.49 (32.57) | 13.19# (6.1-20.3) |
Data expressed as Mean (SD). ICS- Inhaled Corticosteroids; FeNO-Fractional exhaled Nitric Oxide; ppb - parts per billion; $p<0.0001; #p=0.001.
Receiver operating characteristics roc curve for asthma severity prediction.
Receiver Operating Characteristics (ROC) curve for Poor Inhaled corticosteroid ICS response prediction.
Predictive values of FeNO for asthma severity and ICS response at various cut-off range.
| Time period | Cut off levels of FeNO (ppb) | Asthma severity prediction | ICS response prediction |
|---|
| Sensitivity | Specificity | Sensitivity | Specificity |
|---|
| Baseline | <25 | 97.9 | 3.70 | 97.0 | 1.4 |
| >25 | 87.5 | 14.8 | 87.9 | 14.5 |
| >50 | 66.7 | 24.1 | 69.7 | 27.5 |
| >75 | 54.2 | 46.3 | 60.6 | 49.3 |
| >100 | 31.3 | 79.6 | 30.3 | 76.8 |
| >125 | 2.10 | 100.0 | 3.0 | 100.0 |
| Follow-Up | <25 | 97.9 | 11.1 | 87.9 | 4.30 |
| >25 | 87.5 | 18.5 | 84.8 | 15.9 |
| >50 | 64.6 | 38.9 | 72.7 | 42.0 |
| >75 | 39.6 | 75.9 | 39.4 | 72.5 |
| >100 | 4.20 | 94.4 | 9.10 | 97.1 |
| >125 | 2.10 | 100.0 | 3.0 | 100.0 |
Data analysed using Receiver Operating Characteristics curve.
Discussion
Overall, in the present study, significant difference in mean FeNO levels was found in mild, moderate and moderately severe asthmatics groups following ICS treatment in spite of having similar baseline FeNO levels. Similarly, significant difference in mean FeNO levels was found in both ICS responders groups after treatment, with similar baseline values. Moreover, the AUC confirms the insignificant predictive association of FeNO with both disease severity and ICS response. Thus, the present study results suggest that FeNO may not act as a predictor of disease severity or future ICS response. It has to be validated with larger sample size and longer ICS treatment duration.
In ADEPT (Airways Disease Endotyping for Personalized Therapeutics), a recent longitudinal study on asthmatics, no significant difference in FeNO levels was found between mild, moderate and severe asthma severity groups but the definitions for asthma severity were deviated from accepted definitions [21]. But the present study findings support the use of FeNO in severity assessment. In a recent cohort study, FeNO values were found to be significantly higher (≥31.5 ppb) in Corticosteroid Responsive Cough (CRC) than in Non-Corticosteroids Responsive Cough (NCRC) [22]. In the present study, though significant decrease in FeNO was observed on treatment, the decrease was not clinically significant to differentiate ICS response groups. In a study to evaluate the influence of the practice setting on diagnostic accuracy of FeNO for diagnosing asthma; and to develop prediction rules for diagnostic decision-making including Clinical Signs and Symptoms (CSS), FeNO was found to appear more effective for ruling in asthma than for ruling it out [23].
Previous studies on the diagnostic accuracy of blood eosinophil, FeNO and serum periostin to assess eosinophilic airway inflammation have demonstrated conflicting results. The results of various studies on FeNO and its correlation with airway inflammation, ICS response, etc., are discussed in detail as summarized in [Table/Fig-8] [12,13,21,24,25]. In a study to investigate the sensitivity and specificity of the above triad variables, blood eosinophil count and FeNO levels were found to detect and distinguish eosinophilic from non-eosinophilic airway inflammation, even in severe asthmatics [24]. In BASALT (Best Adjustment Strategy for Asthma in the Long Term) RCT, Physician-, Biomarker-, and Symptom-Based Strategies for Adjustment of Inhaled Corticosteroid Therapy in Adult asthmatics were compared and either biomarker-based or symptom-based adjustment of ICS was found not to be superior to physician assessment–based adjustment of ICS in time to treatment failure [25].
Studies on association of FeNO, as a biomarker with other clinical parameters [12,13,21,24,25].
| Definition of outcomes | Time to outcome (mths) | Subjectsn | Age(yrs) | Clinical parameters | Results | First author (Ref) |
|---|
| Asthma control level | - | 363 | 46.3 | History, physical examination, BMI, PFT, ACT and FeNO | FeNO levels associated with poorly controlled asthma | Ricciardolo [12] |
| ACOS prevalence with high FeNO levels | - | High FeNO,n=331;Low FeNO,n=230 | >70 | PFT, IgE and FeNO | 16.3% prevalence rate of ACOS with FENO>35 ppb | Tamada[13] |
| Asthma profiling to correlate clinical features and biomarkers with molecular characteristics | 12 | 150 (50 in each-mild, moderate, severe); 30 controls | 18–55 | History, patient questionnaires, PFT, hyper-responsiveness FENO, biomarkers and bronchoscopy | FENO andblood eosinophils did not differ by asthma severity | Silkoff[21] |
| Relationship ofblood eosinophils, FeNO, and serum periostin with sputumeosinophils | - | External validationcohort, n=110; Replication cohort, n=37 | 49&53 | Sputum induced eosinophils, blood eosinophil counts, serum periostin and FeNO | FENO, second-best predictor for eosinophilic inflammation, but no additive value with blood eosinophils | Wagener[24] |
| BASALT trial | 9 | 342 in3-groups:(n = 114, physician assessment–based; n = 115,FeNO-based; n = 113 symptom-based | 34.2-36.0 | Time to treatment failure | Use of either FeNO-based or symptom-based adjustment of ICS not superior to physician assessment–based | Calhoun[25] |
| Clinical utility of FeNO as a biomarker to predict disease severity and ICS response in asthmatics | 2 | 102 | 18-50 | PFT and FeNO | Decrease in FeNO levels on ICS treatment not a predictive of asthma severity and ICS response | Present study |
FeNO-Fractional exhaled Nitric Oxide; BMI-Body Mass Index; PFT-Pulmonary Function Test; ACT-Asthma Control Test; ACOS: Asthma–Chronic Obstructive Pulmonary Disease (COPD) Overlap Syndrome; BASALT: Best Adjustment Strategy for Asthma in the Long Term; ICS-Inhaled Corticosteroids
In an Indian study, significantly higher FeNO levels were reported among allergic rhinitis patients [16]. In our study, with considerable number of patients with rhinitis as a co-morbidity condition (n=45), the baseline FeNO levels was found to be elevated in mild, moderate and moderately severe asthmatics groups. In a study to estimate the range of FeNO in Indian patients of Obstructive Airway Diseases (OAD), mean FeNO values were found higher in asthmatics as compared to COPD patients, with 5-300 ppb and 10-47 ppb in the Asthma and COPD groups, respectively [17]. In the present study, overall baseline mean value of FeNO (97.8 ppb) was found elevated in asthmatics consistent with the above findings in asthmatics group.
With reduced number of smokers and alcoholics recruited in the present study, the findings are with minimal confounders since smoking reduces FeNO levels by 30 to 60%. Independent factors such as age, sex, BMI, previous ICS use, etc., has its significant effect size on FeNO levels [26]. In a previous study, no correlation was observed between clinical evaluation tools of asthma management such as Asthma Control Questionnaire (ACQ), Asthma Control Test (ACT), National Asthma Education and Prevention Program (NAEPP), in all age groups [27]. The present study results reports no correlation of baseline FeNO levels with severity or ICS response assessment.
A significant dose-response relationship between ICS and elevated FeNO in asthma patients was observed previously, in shorter treatment duration. Moreover, patients with high FeNO (>50 ppb) were found more likely to respond than those with low FeNO (<50 ppb) [28]. In the present study, differentiation of ICS response in the above manner was not done as there were only 17 patients with low FeNO level. But the ICS treatment duration followed in the present study (8 weeks) is quite considerable to assess response phenotype compared to other previous reports.
Our study results are in line with a previous study where FeNO was unable to significantly predict lung function improvement after treatment with beclomethasone equivalents. Reported AUC for ROC for predicting FEV1 improvement based on baseline FeNO levels was 57% [29]. In our study, the AUC for ROC was 56.1% and 58.0% for baseline and follow-up FeNO levels in ICS response prediction. Moreover, the treatment duration in the present study was 8 weeks and both baseline and follow-up FeNO levels were analysed. The present study results are in line with the findings PRICE trial, with similar levels of FeNO in both ICS response groups defined by percent improvement in FEV1 [30].
To the best of our knowledge, the present study is first of its kind in reporting the clinical use of FeNO as a diagnostic biomarker in disease severity prediction and also in drug response assessment. This study adds to the present knowledge on clinical utility of FeNO in Indian scenario, as only few Indian studies are available reporting FeNO use in asthma diagnosis. Moreover, this is the first study reporting clinical use of FeNO measurements in severity assessment and ICS response prediction with respect to specific ethnicity or geographical distribution, i.e., in Tamilian population residing in Tamilnadu or Puducherry. The present study mainly depends on PFT parameters to sub-group the study population based on disease severity and ICS response. These PFT parameters are ethnicity specific and the reference values differ between South Indians and North Indians. Thus, we are interested to specify the ethnicity of study population. Though the present sample size do not represent overall Tamilian population, this study is first of its kind in Tamilians and may help as a preliminary work in planning future studies in asthma research.
Conclusion
Thus, we conclude that though the present study results do not support the predictive association of baseline FeNO levels with asthma severity and ICS response, the decrements in FeNO levels on ICS treatment, supports its clinical utility in monitoring of ongoing airway inflammation and understanding treatment response rate. FeNO monitoring in prospective studies with larger sample size and longer follow-up duration (>8 weeks) compared to the present study helps us to understand better the predictive association of FeNO as a diagnostic biomarker of both disease severity and ICS response.
Inferences from systematic reviews, Global Initiative for Asthma (GINA) and ATS guidelines recommend the use of FeNO in clinical practice and asthma diagnosis but the recommendations are not strong due to heterogeneity in study reports require further research. FeNO monitoring in routine clinical practice may be a useful tool in assessing asthma control along with other clinical factors in future. Further studies are warranted on this specific ‘asthma phenotype’ considering long-term ICS treatment outcomes and frequency of exacerbations in all age groups.
Data expressed as Mean (SD); ED-Emergency department; OCS-Oral Corticosteroids
FN: Data expressed as Mean (SD). Data analysed using paired t-test. BD-Bronchodilation with Salbutamol nebulization; FVC - Forced Vital Capacity (l); FEV1 - Forced Expiratory Volume in 1.0 second (l); PEFR- Peak Expiratory Flow Rate (l/s); FEF 25-75%- Forced Expiratory Flow Rate at 25-75%.*p<0.05.
FN: Data expressed as Mean (SD). Data analysed using paired t-test. p<0.05 considered as significant. ICS-Inhaled Corticosteroids; CI-Confidence Interval; FVC - Forced Vital Capacity (l); FEV1 - Forced Expiratory Volume in 1.0 second (l); PEFR- Peak Expiratory Flow Rate (l/s); FEF 25-75%- Forced Expiratory Flow 25-75%.*p<0.0001
Data expressed as Mean (SD). ICS- Inhaled Corticosteroids; FeNO-Fractional exhaled Nitric Oxide; ppb - parts per billion; $p<0.0001; #p=0.001.
Data analysed using Receiver Operating Characteristics curve.
FeNO-Fractional exhaled Nitric Oxide; BMI-Body Mass Index; PFT-Pulmonary Function Test; ACT-Asthma Control Test; ACOS: Asthma–Chronic Obstructive Pulmonary Disease (COPD) Overlap Syndrome; BASALT: Best Adjustment Strategy for Asthma in the Long Term; ICS-Inhaled Corticosteroids