Case Report
A 10-year-old child presented to paediatric Outpatient Department (OPD) with a solitary, small, papulonodular swelling of size 2×2 cm in the labia majora since past 2 years. The lesion was insidious in onset, progressively increasing in size, however not painful. On examination, it was firm, mobile, non-tender with well-defined margins. No other similar lesions were present anywhere else in the body. Based on the age of the patient, a provisional clinical diagnosis of langerhans cell histiocytosis was kept and the patient was subjected to further investigations.
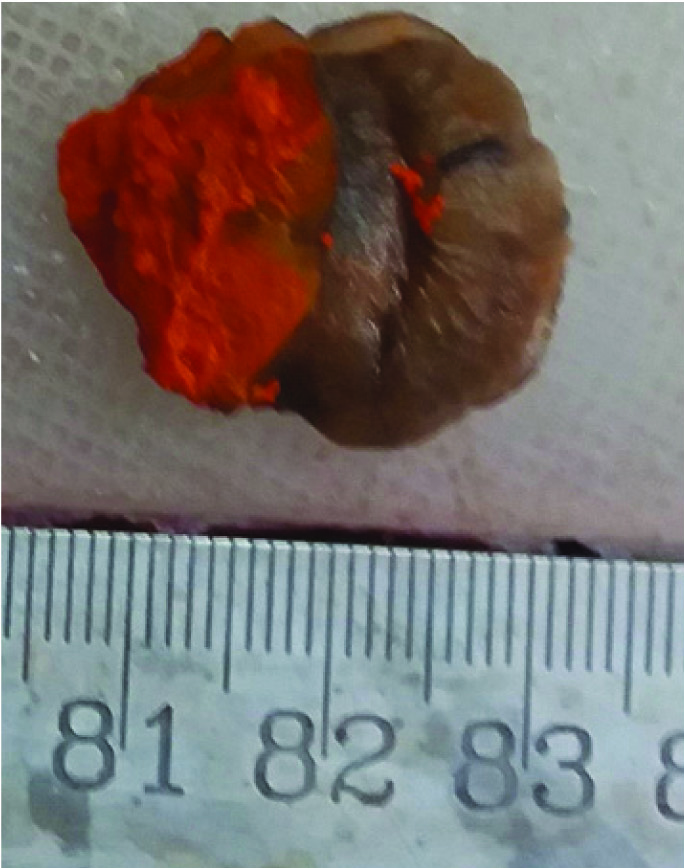
Fine Needle Aspiration Cytology (FNAC) yielded a scanty, inadequate aspirate. Excision biopsy was performed and sent for histopathological examination which revealed a skin covered polypoidal tissue bit measuring 2×2 cm. On cut section, the lesion was firm, grey-white in color [Table/Fig-1]. Microscopic examination showed an unremarkable epidermis. Underlying dermis showed an unencapsulated, cellular lesion comprising of oval to spindle shaped cells arranged in sheets and vague storiform pattern admixed with multinucleated touton giant cells. Individual cells showed vesicular, irregular nuclei, inconspicuous nucleoli and moderate to abundant amount of eosinophilic cytoplasm with few cells showing foamy cytoplasm [Table/Fig-2]. Interspersed chronic inflammatory infiltrate comprising of eosinophils and histiocytes was noted [Table/Fig-3]. Cells showed infrequent mitosis but lacked atypia. No area of necrosis was noted. On immunohistochemistry, the mononuclear cells and giant cells were positive for vimentin and CD68 and negative for CD1a and S-100 protein [Table/Fig-4]. The findings were consistent with classic Juvenile Xanthogranuloma (JXG). Deep resected plane and both lateral margins were 0.2 cm away. The patient had an uneventful clinical outcome, however was lost to follow-up.
Gross: Skin covered polypoidal tissue bit.
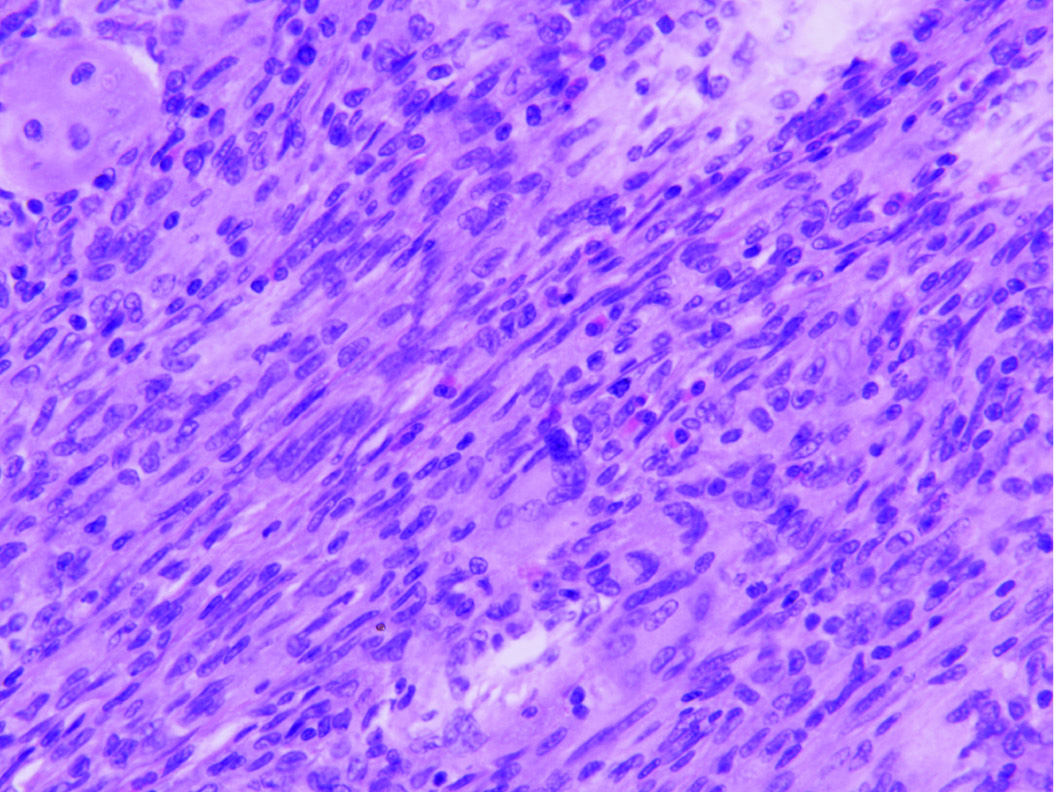
Shows cellular lesion in dermis comprising of oval to spindle shaped cells arranged in sheets and vague storiform pattern admixed with Touton-type giant cells (H&E, 20X).
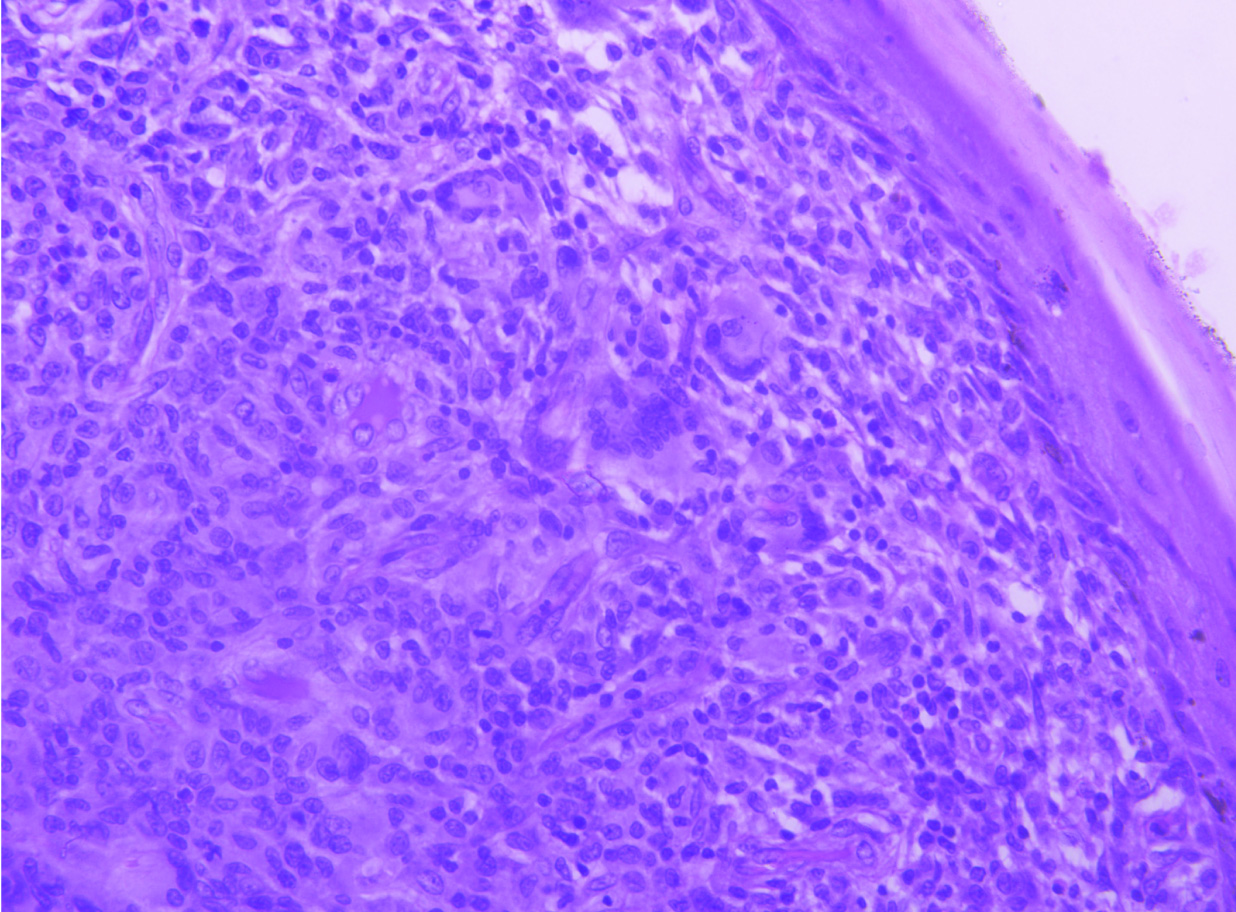
Interspersed chronic inflammatory infiltrate rich in eosinophils and histiocytes (H&E, 40X).
(a) Mononuclear cells and giant cells showing cytoplasmic positivity with CD68 and (4X); (b) negative for CD1a (10X).

Discussion
JXG is a histiocytic disorder, derived from accumulation or proliferation of cells derived from the dermal dendrocyte [1] and presents as a solitary or multiple papulo-nodular lesions mostly involving head and neck region. Cases have been reported in various internal organs such as lungs, bones, genitalia, heart, eyes and gastro-intestinal tract [1,2], however, there are only occasional case reports of xanthogranuloma involving vulva in adult age group [3–5]. Though the lesion in children, unlike adults, is asymptomatic and self-regressing [6], the site becomes a cause of concern for the parents. Excision is often advised to arrive at a diagnosis.
JXG shows three characteristic histologic patterns: Early JXG (EJXG), classic JXG (CJXG), and transitional JXG (TJXG) [7]. EJXG is characterized by sheets of small to intermediate-sized mononuclear histiocytes with little lipid. Touton-type giant cells are absent. Cells lack atypia however show slightly more mitotis. CJXG exhibits characteristic appearance of JXG with presence of abundant vacuolated, foamy histiocytes and touton giant cells. TJXG shows spindle-shaped cells arranged predominantly in a storiform pattern resembling Benign Fibrous Histiocytoma (BFH) with foamy histiocytes, inflammatory infiltrate rich in eosinophils and occasional giant cells [7].
Histology in the present case matched with the CJXG pattern with the presence of sheets of spindle shaped cells arranged in vague storiform pattern admixed with touton giant cells, eosinophils and histiocytes. Though infrequent mitotis was seen, no atypia/ necrosis were noted.
Differential diagnosis includes both benign and malignant lesions which present either in the same paediatric age group and have been known to occur in vulva or mimic JXG histologically.
Langerhans Cell Histiocytosis (LCH) is the first and the foremost differential diagnosis of JXG and should be ruled out in all cases [7]. LC can be found in the epidermis and mucosal lining of multiple organs including cervix, vagina, stomach and esophagus [8]. Even rare case reports of pure vulval LCH also exist in the literature [9]. The lesion presents as solitary or multiple papules or nodules making them sometimes clinically indistinguishable from JXG like in the present case.
Histological diagnosis is often necessary and the lesion shows presence of LC characterized by lobulated, eccentric, grooved vesicular nuclei with coffee bean appearance, inconspicuous nucleoli and abundant eosinophilic cytoplasm admixed with variable inflammatory infiltrate comprising of neutrophils, lymphocytes, plasma cells and eosinophils. In some cases, LC can resemble touton giant cells. IHC for CD1a, S-100 and langerin can be performed in cases of doubt [8]. Characteristic intra-cytoplasmic Birbeck granules as seen by electron microscopy are seldom required for diagnosis. In the present case, diagnosis of LCH was ruled out by the absence of LC on morphology. This was further confirmed by IHC with negative S-100 and CD1a.
Other differential diagnosis includes fibrohistocytic lesions NOS which show presence of spindle cells arranged in storiform pattern with histiocytes and giant cells, however, they usually lack eosinophilic infiltrate which was present in this case.
Reticulohistiocytoma, non-LCH group of histiocytic disorder, should also be considered in differential diagnosis due to its histological resemblance to JXG. It is characterized by large mononucleated or multinucleated histiocytes along with lymphocytic inflammatory infiltrate and dermal fibrosis. On IHC, they were positive for CD68 and negative for S-100 and vimentin, unlike JXG, which is positive for vimentin.
Common nevi, epidermal and dermal nevi all fall in the category of differential diagnosis however can easily be distinguished from JXG based on their morphology. Spitz nevus can be other rare possible differential diagnosis of JXG sharing a common paediatric age group and site. One such case has been reported in 11-year-old girl in vulva [10]. Histologically, tumor lacks melanin pigment and comprises large cells with vesicular nuclei, intranuclear pesudoinclusions, prominent nucleoli and abundant cytoplasm. Lesion can be easily distinguished from JXG based on its morphology.
Rarely infantile haemangioendothelioma has been reported in a nine-month-old female in vulva [11]. The lesion however differs markedly in its histological appearance and shows presence of lobules of variable sized capillaries lined by bland endothelial lining, intermixed with scanty stroma with occasional focal areas of necrosis.
Malignant neoplasms such as Malignant Fibrous Histiocytoma (MFH) [12] and Embryonal Rhabdyosarcoma (ER) [13], both of which have been known to present as vulval nodules, should also be kept in mind as differential diagnosis. Whereas, MFH occurs in an elderly age group and shows marked degree of pleomorphism, mitosis, necrosis and tumor giant cells, JXG totally lacks atypia. Though ER shares a common paediatric age group with JXG, proliferation of monomorphic small round cells is seen in it with presence of cambium layer and necrosis.
JXG presenting as a vulval nodule is extremely rare and extensive search of the existing literature revealed only handful of cases reported in adult age group [3–5]. Though Campourcy M et al., reported a cutaneous nodule in an eighteen month old child in labia majora and chin; the nodule showed presence of CD 68 positive histiocytes, however, lacked giant cells. Ultrastructurally, few cells showed features of LCH and therefore a final diagnosis of acquired, regressive LCH was rendered without further categorization [14]. Rodriguez and Ackerman described xanthogranulomas at multiple locations with lesions on the face, scalp, axilla and genitalia however further site of origin were not specified in their published article [15].
To the author’s best knowledge, this is the first case of JXG being reported as vulval nodule in a paediatric age group. Though solitary JXG are well known, JXG is known to be rarely associated with other diseases like neurofibromatosis, juvenile myelomonocytic leukaemia, niemann-pick disease and urticarial pigmentosa. This possibility though rare, should not be ignored and a thorough workup of a patient diagnosed with JXG must be done to rule out the possibility of co-existent pathology. In our case, the patient was lost to follow up and detailed workup could not be done.
Conclusion
Though vulval JXG has been occasionally reported in adults, this is a first case report of solitary, vulval JXG in a paediatric age group. The lesion is rare and, unlike adults, is self-regressive in children and thus harbours a favourable prognosis and should be kept in mind as one of the differential diagnosis of vulval nodule.
[1]. Dehner LP, Juvenile xanthogranulomas in the first two decades of life: a clinicopathologic study of 174 cases with cutaneous and extra cutaneous manifestationsAm J Surg Pathol 2003 27(5):579-93. [Google Scholar]
[2]. Freyer DR, Kennedy R, Bostrom BC, Kohut G, Dehner LP, Juvenile xathogranuloma; forms of systemic disease and their clinical implicationsJ Pediatr 1996 129(2):227-37. [Google Scholar]
[3]. Castillo EA, Ormsby – Adult xanthogranuloma of the vulva: case report and reviewPathology 2002 34:86-87. [Google Scholar]
[4]. DeLuca IJ, Grossman ME, Vulvar necrobiotic xanthogranulomaJournal of the American Academy of Dermatology 2014 71(6):e247-48. [Google Scholar]
[5]. Tran DT, Wolgamot GM, Olerud J, Hurst S, Argenyi Z, An ‘eruptive’ variant of juvenile xanthogranuloma associated with langerhans cell histiocytosisJournal of Cutaneous Pathology 2008 35:50-55. [Google Scholar]
[6]. Jung T, Emmert S, Gunzl JJ, Neumann C, Runger TM, Congenital manifestations of juvenile xanthogranuloma (large nodular form)Hautarzt 2000 51(6):423-26. [Google Scholar]
[7]. Janssen D, Harms D, Juvenile xanthogranuloma in childhood and adolescence: a clinicopathologic study of 129 patients from the kiel pediatric tumor registryAm. J Surg Pathol 2005 29:21-28. [Google Scholar]
[8]. Park HJ, Jeon YK, Lee AH, Oh YH, Park SH, Jung KC, Use of the JL1 epitope, which encompasses the nonglycosylation site of CD43, as a marker of immature/neoplastic Langerhans cellsAm J Surg Pathol 2012 36(8):1150-57. [Google Scholar]
[9]. Triantafyllidou O, Giannakopoulos K, Pergialiotis V, Simou M, Lagkadas A, Alexandrou P, Pure vulvar Langerhans cell histiocytosis: a case report and literature reviewEur J Gynaecol Oncol 2009 30(6):691-94. [Google Scholar]
[10]. Polat M, Topcuoglu MA, Tahtaci Y, Hapa A, Yilmaz F, Spitz nevus of the genital mucosaIndian J Dermatol Venereol Leprol 2009 75(2):167-69. [Google Scholar]
[11]. Elek Z, Radosavljevic Z, Turkovic B, Labia majora haemangioendothelioma – case reportActa medica medianae 2007 4:74-76. [Google Scholar]
[12]. Santala M, Suonio S, Syrjanen K, Uronen MT, Saarikoski S, Malignant fibrous histiocytoma of the vulvaGynecologic Oncology 1987 27(1):121-26. [Google Scholar]
[13]. Youngstrom EA, Bartkowski DP, Vulvar embryonal rhabdomyosarcoma: A case reportJournal of Pediatric Urology 2013 9(4):e144-46. [Google Scholar]
[14]. Campourcy M, Moreau-Cabarrot A, Gorguet B, Samalens G, Daste B, Eclache F, Histiocytose cutanee acquise regressive non langerhansienne de l’enfant. (Acquired regressive cutaneous non-Langerhans’-cell histiocytosis in an infant.)Ann Dermatol Venereol 1997 124:167-70. [Google Scholar]
[15]. Rodriguez J, Ackerman B, Xanthogranuloma in adultsArch Dermatol 1976 112:43-44. [Google Scholar]