Chronic Obstructive Pulmonary Disease (COPD) is a heterogeneous disease which is characterized by persistent and irreversible airflow limitation, with numerous extra-pulmonary complications; co-morbidities, impaired quality of life and increased mortality. It is the fourth-leading cause of chronic morbidity and mortality worldwide [1]. Prevalence is expected to increase further due to smoke exposure and the changing age structure of the world population. It is accompanied by chronic inflammation of the airways and lung parenchyma [2]. Although COPD mainly affect persons in the age group of 40–60years, the pathogenesis of this disease can begin in early life and may lead to early death [3,4].
Total Homocysteine (tHcy), is a 4-carbon amino acid attached to a sulphydryl group. Hcy is involved in the transfer of methyl groups and is an intermediate product of the metabolism of methionine [5]. There is a growing body of evidence that suggests COPD to be an independent risk factor for atherothrombosis. The incidence of mild-to-moderate hyperhomocysteinemia is relatively common in the general population, mainly associated with deficiencies of vitamin B, which constitutes essential cofactors in tHcy catabolism [6]. Smoking remains the most important risk factor in the development of COPD and vascular disease and cigarette smoking causes elevation of plasma Hcy [7–10] though the effect may be variable. Several studies have reported and linked Hcy with cardiovascular risk [11–13]. Since both cardiovascular disease and COPD share a common cause [14] which itself causes hyperhomocysteinemia, it is reasonable to expect that COPD should be associated with elevated Hcy. There has hitherto not been much interest in Hcy disorders in respiratory disease. There has been only a few small sample sized studies done, investigating the role of Hcy in COPD patients till date. Several studies have reported an increased level of tHcy in COPD patients compared to healthy subjects [8,15–17].
Vitamin B12 and folic acid plays an important role in the metabolism of Hcy. Study done in the past showed reduction in Hcy by increased intake of folate or vitamin B12 was not associated with decreased serum inflammatory markers or cardiac events. However, raised serum folate may have direct effects at multiple sites including atherosclerotic plaques and thus, other methods of reducing tHcy may yield different results on serum inflammatory markers [18–20]. Since COPD is a disease which is characterized by complex nutritional abnormalities and lower levels of vitamin B12 and folic acid, may lead to elevated Hcy levels.
Till date, the status of folic acid supplementation and its effect on hyperhomocysteinemia in COPD have never been investigated and there is a paucity of data about hyperhomocysteinemia in patients with stable COPD in Indian population. Against this background, in the current study, we estimated the plasma levels of tHcy in patients with COPD and matched healthy control subjects to determine the correlation of hyperhomocysteinemia with severity of disease and effect of folic acid supplementation if any.
Materials and Methods
The study protocol conforms to the principles of the Declaration of Helsinki and was approved by the Institutional Ethical Committee of Maulana Azad Medical College and associated Lok Nayak Hospital, New Delhi, India. Written informed consent was obtained from all subjects prior to the participation. COPD patients attending the chest clinic and Medicine Outpatient department were recruited from August 2011 to February 2013, whereas asymptomatic healthy controls, irrespective of smoking habits, were among the patient’s attendants visiting the hospital. Asymptomatic controls were defined as persons over the age of 40 years without any known medical illness and who were symptom free.
Lung function tests were performed in all subjects using standardized methods according to the American Thoracic Society guidelines [21,22]. All study participants were examined by a study physician who performed a structured interview of the medical history including exacerbations, co-morbidities, smoking and medication use. Clinical diagnosis of COPD and inclusion criteria were: (a) post-bronchodilator FEV1/FVC<70%; (b) reversibility of FVC or FEV1, induced by β-agonist (200mg salbutamol) <12% or 200 ml; (c) no exacerbation within the last 12 weeks before recruitment. Patients were excluded if they had a diagnosis or history of asthma and conditions known to affect serum levels of Hcy, including coronary heart disease and metabolic syndrome.
Patients were subjected to complete history and physical exami-nation and particulars of the patients such as name, age, sex, pack years of smoking etc., were noted in a pre-structured performa. Baseline spirometry, chest X-ray and ECG were done. A series of hematological and biochemical investigations i.e., hemogram, liver function test, kidney function test, fasting blood sugar and lipid profile were carried out. Anthropometric measurements (height, weight) were also done to calculate Body Mass Index (BMI). All patients were given folic acid therapy (5mg daily) for duration of six weeks. Patients were followed up and repeat plasma Hcy and FEV1 were measured after six week of folic acid supplementation [Table/Fig-1].
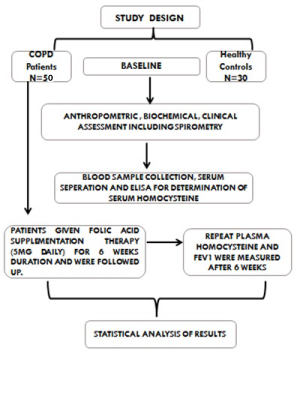
Blood Sampling: Subjects were asked to fast overnight from 21:00 the night before, after which venous blood samples were collected and serum was separated immediately and stored at -20°C until analysis. Levels of plasma Hcy were analyzed using an ELISA [(The Axis® Homocysteine Enzyme Immunoassay (EIA) kit 9 (United Kingdom)] in the Department of Biochemistry, Maulana Azad Medical College, New Delhi, India. All the measurements were carried out strictly according to the manufacturer’s instructions.
Statistical Analysis
All statistical analyses were performed by SPSS 18.0 for Windows (IBM, Chicago, IL). Variables with skewed distributions were log transformed before further analysis. All continuous data were presented as mean ± standard deviation (SD), whereas categorical data were presented as frequency and percent. Comparisons of characteristics between groups were performed by unpaired Student’s t or Chi-square tests when appropriate. Pearson’s correlation adjusting for age, BMI, pack-years smoked, and current smoking status was used to analyze the correlations between homocysteine and lung function. The level of significance was set as p<0.05.
Results
Patient’s Characteristics at the baseline: A total of 50 COPD patients and 30 healthy controls were enrolled in this study. All the demographic data and baseline characteristics of the study subjects are summarized in [Table/Fig-2]. Out of 50 COPD patients 86% were male while 14% were female, whereas in control group male and female were 43.3% and 56.7% respectively. The mean BMI of the COPD patients was 21.3±4.26 with range from 16 to 29.8. More than 80% of patients had BMI <25kg/m2 at the time of enrollment. There were no differences with respect to the age and BMI between the COPD patients and the controls. Among 50 COPD patients 76% were smokers while 24% non-smokers. There were significant reductions in FEV1% predicted and FEV1/FVC ratio compared with healthy controls. Meanwhile, plasma level of tHcy was significantly elevated in COPD patients compared with healthy controls (27.42±23.89 vs. 15.21±15.71, p<0.001) [Table/Fig-2].
Baseline demographical data, lung function, and laboratory variables of the COPD patients and controls.
| Parameters | COPD(n=50) | Controls (n=30) | p-value |
|---|
| Age (yrs) | 58.3±9.2 | 51.5±7.6 | 0.004 |
| Male/Female | 43/7 | 13/17 | - |
| Smoking (Status) |
| Smoker (yes) | 38 (76%) | 16 (53.3%) | - |
| Non smoker | 12 (24%) | 14 (46.6%) | - |
| Pack years | 33.6±17.4 | 14.4±9.5 | <0.001 |
| BMI (Kg/m2) | 21.36± 4.2 | 22.8±4.6 | 0.42 |
| FEV1(%Predicted) | 1.14±0.60 | 1.08±0.56 | 0.6546 |
| FEV1/FVC (%) | 1.62±6.70 | 1.50±4.62 | 0.8992 |
| FEV1 Reversibility (%) | 11.7±10.4 | 5.5± 4.8 | <0.001 |
| FVC (%) | 89.4±16.6 | 110.4±13.5 | 0.004 |
| PO2 mmHg | 62.5±11.4 | 89.6±14.8 | <0.001 |
| PCO2 mmHg | 41.6±9.8 | 38.6±3.8 | 0.024 |
| Total cholesterol mg/dl | 237.5± 37.50 | 223.4±35.12 | 0.042 |
| HDL, mg/dl | 43.2±12.4 | 47.6±13.6 | 0.247 |
| LDL, mg/dl | 133.4±24.8 | 129.4±19.6 | 0.341 |
| Total Homocysteine (tHCY) ìmol/L | 27.42±23.89 | 15.21±15.71 | <0.001 |
Abbreviations: BMI-Body mass index; FEV1-forced expiratory volume in the first second; FVC-forced vital capacity; HDL-High Density Lipoprotein; LDL-Low density lipoprotein; PO2-Partial pressure of oxygen; PCO2-Partial pressure of carbon dioxide; tHCY-Total homocysteine
In COPD group 16% had normal Hcy level while, 68% moderate, 14% intermediate, and 2% had severe hyperhomocysteinemia. On the other hand among the control group 60% had normal Hcy levels and 36.6% moderate, 3.34 %intermediate, none had severe hyperhomocysteinemia. Those patients who were smokers had higher homocysteine levels with mean 28.3±26.7μmol/L as compared to non-smokers 24.6±11.17μmol/L (p-value=0.64). Also, the mean FEV1 was lower (0.99±0.48) as compared to non- smokers (1.06±0.43) (p-value=0.66).
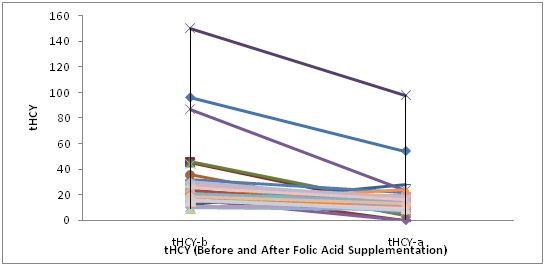
Homocysteine, lung function and BMI after six weeks of folic acid supplementation: In our study folic acid treatment did not have significant affect on pulmonary function parameters in COPD patients. The mean FEV1 value before and after supplementation were 1.14±0.60 and 1.08±0.56 respectively with p-value=0.534. The difference was statistically non-significant. On the other hand, the mean Hcy values decreased significantly from 27.42±23.89μmol/L to 15.2±15.71μmol/L after folic acid supplementation respectively (p-value<0.001) [Table/Fig-3].
Comparison of FEV1 and tHCY before and after folic acid supple-mentation.
| Parameters | COPD (N=50) | 95% Confidence Interval of Difference | p-value |
|---|
| tHCY- (Before) | 27.42±23.89 | -18.20 to -8.885 | <0.001 |
| tHcy- (After) | 15.21±15.71 |
| FEV1% (Before) | 1.14±0.60 | -0.20 to 0.105 | 0.534 |
| FEV1% (After) | 1.08±0.56 |
Abbreviations: FEV1-Forced expiratory volume in the first second; tHcy-Total homocysteine
At the baseline and after six weeks of folic supplementation Hcy exhibited a weak and non-significant correlation with FEV1 (r=0.125, p=0.43 and r=0.188 p=0.23, respectively), and BMI also weakly correlated with FEV1 at the baseline and after folic acid supplementation (r=0.073, p=0.64 and r=0.085, p=0.56) in crude analyses. The correlation was non-significant [Table/Fig-4]. However, a significant correlation was observed between BMI and Hcy at the baseline and after six weeks of folic acid supplementation (r=0.283, p=0.04 and r=0.340, p=0.02 respectively). We also found a strong positive and significant correlation between plasma Hcy levels at the baseline and after six weeks of folic acid supplementation (r=0.840 p=0.001) [Table/Fig-4,5].
Correlation of homocysteine with body mass index and pulmonary function parameter (before and after comparison).
| Parameters | Correlation | BMI | FEV1% (b) | FEV1% (a) | tHcy(b) | tHcy (a) |
|---|
| BMI | Pearson’s Correlation | 1 | 0.073 | 0.085 | 0.283* | 0.340* |
| Sig. (2-tailed) | | 0.646 | 0.560 | 0.046 | 0.028 |
| FEV1(b) | Pearson’s Correlation | 0.073 | 1 | 0.657** | 0.125 | 0.188 |
| Sig. (2-tailed) | 0.646 | | 0.001 | 0.430 | 0.232 |
| FEV1 (a) | Pearson’s Correlation | 0.085 | 0.657** | 1 | 0.144 | 0.248 |
| Sig. (2-tailed) | 0.560 | 0.001 | | 0.319 | 0.113 |
| tHcy(b) | Pearson’s Correlation | 0.283* | 0.125 | 0.144 | 1 | 0.840** |
| Sig. (2-tailed) | 0.046 | 0.430 | 0.319 | | 0.001 |
| tHcy (a) | Pearson’s Correlation | 0.340* | 0.188 | 0.248 | 0.840** | 1 |
| Sig. (2-tailed) | 0.028 | 0.232 | 0.113 | 0.001 | |
**Correlation is significant at the 0.01 level (2-tailed)
*Correlation is significant at the 0.05 level (2-tailed)
Abbreviations: BMI-Body mass index; FEV1-forced expiratory volume in the first second; tHcy-Total homocysteine
Total homocysteine levels at the baseline and after six weeks of folic acid supplementation in the COPD group.
A significant reduction was observed in plasma tHcy levels after six weeks of folic acid supplementation (p<0.001).
Discussion
The possible pathogenic mechanism of elevated concentrations of Hcy in lung disease remains unclear. Elevated concentrations of Hcy and decreased concentrations of folate may have other pro-inflammatory properties in the COPD patients but their amelioration through folic acid supplementation is likely to influence concentrations of Hcy in such patients.
The possible pathogenic mechanism remains unsolved and convincing evidence to support a causal relationship between elevated concentrations of Hcy and risk of developing COPD is absent. In this study, we observed a marked reduction of elevated Hcy concentrations through daily folic acid supplementation for six weeks duration however; it did not affect the pulmonary function parameters in COPD patients. Our data strongly suggests that hyperhomocysteinemia may result from poor folic acid status in COPD patients which are recognized co-factors in tHcy degradation [5]. To the best of our knowledge this was the first study which looked into the status of folic acid supplementation and its effect on hyperhomocysteinemia and severity of disease in COPD patients in Indian population.
In our study mean baseline values of tHcy were highly variable with range from 9 to 150. The mean value of Hcy in COPD patients was 27.42±23.89μmol/L as compared to control which had a mean of 15.21±15.71 μmol/L (p-value=0.001). Anderson et al., investigated the difference in plasma Hcy in 19 patients with COPD and in 29 healthy subjects [15]. They assessed the influence of pro-oxidant activity on the metabolism of Hcy and found a higher level of tHcy in COPD patients than controls. They observed a decreased concentration of reduced glutathione and a decreased ratio of reduced/total glutathione in COPD patients compared to the healthy individuals. They speculated a possible effect of extracellular pro-oxidant activity on the concentration of total plasma Hcy in patients with COPD. Also, previous studies done by Kai S, et al., (12μmole/L vs. 9.8μmole/ L), Seemungal et al., (12μmole/L vs. 8.2μmole/L) and Fimognari et al., (13.9μmole/L vs. not available) had reported plasma tHcy to be higher in COPD patients than in controls [8,16,17]. The Hcy levels in the COPD patients (27.4±23.85μmol/L) were higher in our study as compared to the above mentioned studies. Also, all of the above studies were relatively small, cross-sectional and done on COPD outpatients and involved a control arm of asymptomatic subjects.
In our study, 82% patients had moderate to intermediate while 2% patients had severe hyperhomocysteinemia among the COPD group as compared to control who had 39.94% (moderate to severe). The Hcy levels were more elevated in COPD stage III-IV (84%) as compared to stage I-II (16%). Similar to our findings, Seemungal et al., also found tHcy was elevated in male COPD patients when compared with female COPD patients, subjects with low FEV1/FVC ratio and low FEV1 % predicted [16]. This study also showed tHcy was higher in patients with GOLD stages III/IV disease as compared with Stages I/II suggesting a role in COPD pathogenesis.
In the current study, we observed a significant decrease in the mean Hcy values from 27.42±23.89 to 15.21±15.71/ L after six weeks folic acid supplementation (p-value<0.001). In cases, relative percent improvement in tHcy among smokers was 39.4±42.5μmol/ L as compared to non-smokers 43.9±36.12μmol/ L (p-value=0.77) after intervention. The mean FEV1 value before and after intervention were 1.14±0.60 and 1.08±0.56 respectively with a non-significant p-value of 0.654. The relative percent improvement in FEV1 in those who were smokers had a mean value of 11.8±33.94 as compared to non-smoker 17.9±26.7 with p-value 0.618 (non-significant). In our study, the mean plasma Hcy declined following six weeks supplementation of folic acid but it was not found to be associated with severity of disease as measured by FEV1. The failure to achieve improvement in FEV1 in our study may be due to the shorter period of supplementation (Six weeks).
Folic acid is the most effective vitamin B to reduce elevated concentrations of Hcy, being responsible for a reduction of approximately 25%. All the previously done studies have found that low folic acid is related to hyperhomocysteinemia [23,24]. A recent case-control study was conducted in central Japan by Fumi Hirayama et al., [25] found that an inverse association was evident between dietary folate intake and the prevalence of breathlessness for adults, together with a significant dose response-relationship for the COPD risk. Moreover, they speculated that increased folate intake might be beneficial to lung function. However, in our study, we did not find any significant change in the lung function. The potential reason which explains the poor folic acid status in COPD patients is unclear. However it is well recognized that in COPD nutritional abnormalities especially of vitamin B group, alternation in caloric intake and decreased BMI complicates the disease [26]. Hence, our results which showed significant decrease in tHcy levels in COPD patients following folic acid supplementation may reflect the underlying micro-nutritional deficiencies that lead to hyperhomocysteinemia in majority of COPD patients. However, whether a dietary folate approach or supplemental folic acid may determine the success of lowering Hcy concentrations still remains unclear.
Limitation
Our study has certain limitations. First being sample size which is relatively small therefore the study may have lacked statistical power. Second, we did not measure the baseline folic acid status in cases and controls and thirdly patients were on standard treatment of COPD, therefore many patients were receiving inhaled steroids and their effect on plasma homocysteine levels is not well understood.
Conclusion
Homocysteine is a ubiquitous amino acid, elevation of which is associated with several diseases as diverse as thrombotic disorders and lung diseases. A strong link between cardiac disease and Hcy levels is already established. The cause and effects of Hcy elevation in COPD are unknown but studies so far available suggest that Hcy is related to COPD pathogenesis and is likely to be associated with alterations in the redox pathway leading to oxidative stress in COPD. However, for now, COPD patients with an elevated Hcy should be screened for cardiac disease and more closely monitored for evidence of a faster decline in lung function. The usage of folate supplements to improve lung function and treatment of COPD and breathlessness symptom should also be investigated in future clinical studies in different population. Possible protective effect of folic acid, director via lowering of Hcy concentrations, may be due to a decrease in inflammation. Lowering of homocysteine concentrations may affect other inflammatory markers, which may affect other pathways leading to COPD. Further prospective cohort studies are recommended to confirm the observed protective effects in other populations and to determine whether increased intakes can improve the mortality rate due to COPD.
Abbreviations: BMI-Body mass index; FEV1-forced expiratory volume in the first second; FVC-forced vital capacity; HDL-High Density Lipoprotein; LDL-Low density lipoprotein; PO2-Partial pressure of oxygen; PCO2-Partial pressure of carbon dioxide; tHCY-Total homocysteine
Abbreviations: FEV1-Forced expiratory volume in the first second; tHcy-Total homocysteine
**Correlation is significant at the 0.01 level (2-tailed)
*Correlation is significant at the 0.05 level (2-tailed)
Abbreviations: BMI-Body mass index; FEV1-forced expiratory volume in the first second; tHcy-Total homocysteine