Introduction
Over the last 100 years, the anatomical location of Bowel Obstruction (BO) has remained unchanged; however, the aetiological factors in small and large BO have changed significantly. With advance of time more and more elderly patients are presenting with BO [1]. But still, BO continues to be one of the most common surgical emergencies [2] encountered in general surgery units and it continues to be a major cause of morbidity and financial expenditure [3]. Peritoneal adhesions and hernia were the most common causes of BO and contributing 42.3% [4]. All patients of BO are potential candidates for major abdominal surgery with long term morbidity and possible mortality. Hence, the decision of surgery and its timing is vital.
Various factors are considered for taking the decision on operative or non-operative management. The factors considered are age of the patients, duration of obstruction, volume of nasogastric aspirate, findings on the radiological imaging, previous abdominal surgeries and malignancy.
Decision in Small Bowel Obstruction (SBO)
Clinical presentation of pain, vomiting, distension and constipation, laboratory and radiographic factors should all be considered when making a decision about treatment of BO [5]. One must rule out an abdominal wall hernia as a cause of BO, which is seen in 26.8% of cases in virgin abdomen [4]. Plain radiograph should be an integral part of management of patients with clinical suspicion of BO and gastrointestinal perforation [6] [Table/Fig-1]. The diagnosis in most cases will be confirmed by further imaging studies such as ultrasound, contrast studies or most commonly in contemporary practice, the Computed Tomography (CT) [7].
Plain X-ray abdomen- localized dilated bowel (Ischemic).
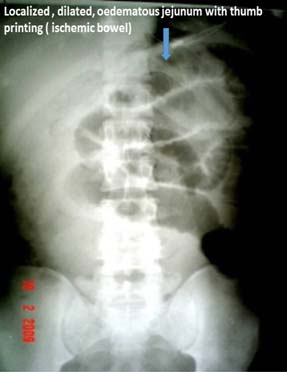
The CT scan, besides confirming the diagnosis of BO, it gives information on partial or complete obstruction, it location, it also provides specific type like closed loop type and helps in deciding early surgery. Contrast Enhanced Computed Tomography (CECT) give enough information on ischemic bowel and bowel oedema, which requires emergency surgery and luminal gastrograffin helps in relieving the BO [8,9].
Surgeons find coronal images more helpful than axial images for management [10]. The radiographic transition zone alone does not increase the likelihood of surgical intervention or identify patients who will fail non-operative management [11]. The four cardinal features – intra peritoneal free fluid, mesenteric oedema, presence of the "small bowel faeces sign" and history of vomiting - are predictive of requiring immediate emergency operative intervention [5].
Decision in large bowel obstruction
In Indian scenario, two common types of Large Bowel Obstructions (LBO) are seen. They are: (a) Acute obstruction due to Sigmoid volvulus (SV); and (b) Sub-acute or chronic obstruction due to cancer of colon. In suspected volvulus, plain X-rays may help with diagnosis but MRI is more reliable. However, flexible endoscopy is always diagnostic as well as therapeutic [12]. Once diagnosed flatus tube, hydrostatic enema or colonoscopic reduction attempt is the choice in vascularized bowel. No endo-luminal reduction to be attempted with the sign of gangrenous SV. If the above methods succeed, later an elective sigmoidopexy or preferably sigmoid colectomy is required to avoid recurrence. When the endoscopic methods fail, emergency laparotomy is indicated, where it is untwisted, the definitive and standard therapy, sigmoid resection and primary anastomosis is the choice [13]. The non-resective alternatives have also been widely used with mixed success, but a large, randomized controlled trial is needed to compare their efficacy with resection and primary anastomosis. Laparoscopic surgery in SV management is unwarranted and costly. Complications of SV include haemorrhagic infarction, perforation, septic shock, and death (14-45%) [14].
Colorectal cancer presents as an emergency with LBO in up to 29% of cases. These patients are often elderly with multiple co-morbidities and deranged physiological function [15].
Almost 42% of such patients will have anaemia preoperatively [16] and showed worse prognosis [17]. Haematological work up and time permitting iron therapy is useful [16,17]. Pre-operative ferric carboxymaltose treatment in patients with colon cancer and iron deficiency anaemia significantly reduced RBC transfusion requirements and hospital length of stay, reaching higher response rates and percentages of normalized haemoglobin levels both at hospital admission and at 30 days post-surgery [16,17]. But, in an emergency setting it may not be of much value.
Due to the deranged physiological setting in elderly, staged surgical procedures were advised, but recent trends have moved towards a primary resection and anastomosis [15].
However, the following options may be considered [18]-
Temporary relief by metal stent, bowel preparation and surgery;
Primary resection and anastomosis- with or without on table preparation;
Initial proximal diversion and staged cancer surgery;
Primary resection of the cancer, loaded proximal colon colectomy, end colostomy and Hartman’s pouch
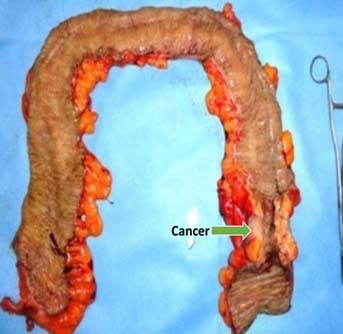
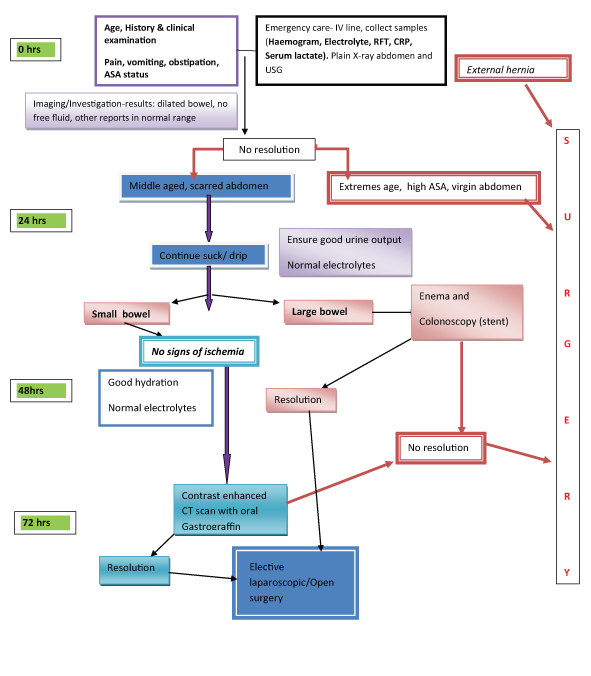
Subtotal colectomy [Table/Fig-2] and ileo-rectal anastomosis.
Subtotal colectomy specimen in left sided cancer colon with obstruction.
Self-Expanding Metal Colonic Stent (SEMS) is found to be safe and effective in obstructing colorectal cancer and effective method of alleviating acute and impending BO [18]. The same can be used in LBO either as palliation or bridge to surgery. They are associated with an overall better outcome and improved quality of life of patients. Surgery is indicated where SEMS are unavailable or have failed [19]. It gives a better chance of primary anastomosis and reduce the need for stoma creation and post procedural complications without any effect on peri-operative morbidity, mortality and long-term survival [20,21]. Delaying the surgery after patency by colonic stent is found to be having higher local recurrence rate in comparison to emergency subtotal colectomy [22].
One stage primary resections with anastomosis of the large bowel can be performed safely in case of emergency whenever patient co-morbidities and local conditions do not stand as major restrictions [23]. Possibly due to gross faecal loading this is not suitable in South East Asia. Options with stomas are not preferred by the patients and also give poor quality of life more so in elderly people. The last option of subtotal colectomy and primary ileo-rectal anastomosis in a semi-emergency basis is a safe and efficient procedure in the management of acutely obstructed neoplasm of the left colon [24]. Despite the difficult pre-operative conditions, subtotal colectomy for left colonic obstruction is found to have lower anastomotic leak rate than the segmental colectomy [25]. The advantages of the last options are many: (a) No bowel preparation, as all the loaded bowel are removed and per anal rectal wash can be given to clean the rectum; (b) It allows to treat in one stage the cancer and the obstruction [24]; (c) Biggest advantage is no stoma; (d) Pre-surgery chronic constipation get relieved; (d) No need of follow up repeated screening colonoscopy; (e) No compromise on the proximal cut margin; (f) Proximal Synchronous lesions are taken care of. The disadvantages are: (a) Technically difficult surgery in presence of grossly dilated and loaded colon [26]. This can be made easier once the mobilized right colon is kept outside the abdomen; (b) Increased frequency of motion for 6-8 months and peri-anal excoriation. In spite of frequent loose motions many patients become happy, because it is a great relief for them after a long period of chronic constipation.
Laparoscopic resectional surgery in acute LBO is feasible and safe option with a low complication rate that enables early hospital discharge [27]. In view of the various alternatives and the lack of high-grade evidence, the treatment of distal colonic obstruction should be individually tailored to each patient [28].
Age related – Decision making in newborn, young and old
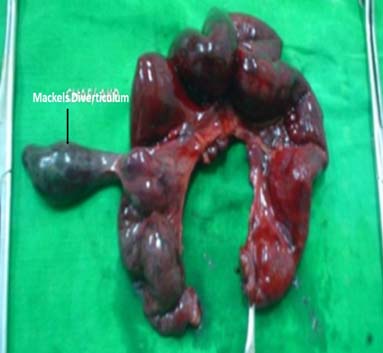
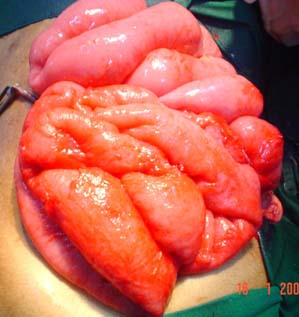
Age is one of the important factors in decision making in BO. Extremes of age have a low body reserve and are liable for higher morbidity and mortality and hence, an early decision is needed for surgery or otherwise [4]. BO in the newborn is a common reason for admission to neonatal ICUs. The incidence is estimated to be approximately 1 in 2000 live births. There are 4 cardinal features of intestinal obstruction in new-borns: (1) Pre-natal maternal polyhydramnios; (2) Bilious vomiting; (3) No passage of meconium; and (4) Abdominal distension. Massive abdominal distension with respiratory distress and cardiovascular collapse is common in neglected megacolon [Table/Fig-3]. A detailed history from the mother and periodic physical examination and X-ray abdomen clinches the diagnosis [29]. Internal hernia and Meckel’s diverticulum related BO [Table/Fig-4] is rare but important. Meckel’s diverticulum can be overlooked in many cases hence, it is recommended that the small bowel be assessed in all cases of appendectomy [30]. 16.4% of children and adolescents undergoing operative management require bowel resections [Table/Fig-5]. To avoid potentially increasing risk for bowel loss, intervention should be considered by the second day in patients who do not exhibit signs of improvement [31].
Meckel’s Diverticulum causing obstruction.
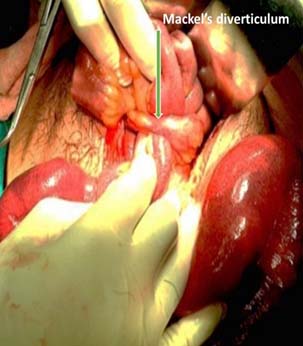
Meckel’s related BO needing resection.
As old age is significantly associated with an increased incidence of strangulation, operative mortality and complications, this group of patients should be managed with extra caution to avoid unfavourable outcome of surgery [4]. Electrolyte imbalance, mostly hyponatraemia and hypokalaemia needs correction before any decision.
Virgin abdomen vs. scarred abdomen
BO in a virgin abdomen is non-adhesive and mostly due to congenital bands and internal hernia. It is rare but, may be a life-threatening surgical emergency. A high index of suspicion based on the patient’s history and response to conservative management is required to achieve early diagnosis so that surgical treatment can be rapidly instituted [32]. Peritoneal adhesions causing SBO is the commonest cause contributing 42.3% [4]. The rate of recurrent obstruction is around 15.9% and 5.8% requiring surgical management [33]. The general impression is that laparoscopic surgery causes less adhesions. But a single centre study has reported no difference in the incidence of post-operative adhesive intestinal obstruction between laparoscopic and open colorectal resection [34]. Surgically treated patients had a lower frequency of recurrence and a longer time interval to recurrence; at the cost of longer hospital stay. There is no predictor of adhesive BO either before or after surgery or any variables predicting the success of a particular treatment [35]. However, patients with matted adhesions have a higher recurrence rate than those with band adhesions. Non-operative treatment for adhesions in stable patients results in a shorter hospital stay and similar recurrence and re-operation rates, but a reduced interval to re-obstruction when compared with operative treatment [3]. These patients need close observation and repeated abdominal examination for evidence of impending bowel ischemia, in the form of raised C-Reactive Protein (CRP) serum lactate, raised total leucocytes count besides guarding.
Recurrent BO
Recurrent bowel obstruction- common causes are given in [Table/Fig-6]. Recurrent BO is seen in three clinical scenario. The first is on an unscarred abdomen, mostly due to internal hernia, secondly during an early post-operative period, where there is likely confusion of post-operative ileus/ BO. The third scenario being adhesive obstruction. All these three situations are discussed below.
Common causes of recurrent bowel obstruction.
| Congenital | Acquired |
|---|
| Internal hernia | Post-operative adhesion |
| Para-duodenal Rt & Lt | Tubercular strictures |
| Para-caecal | Luminal worms/ polyp Bezoars |
| Mesenteric and mesocolic defect hernia | GJ intussusception |
| Cong adhesion | Bezoars |
| Vitelline duct related | Pseudo obstruction |
| Caecal and sigmoid volvulus | Luminal worms/ polyp |
| Radition enteritisEndomatriosisGall stone ileusTalk granuloma related |
a. Recurrent BO in virgin abdomen
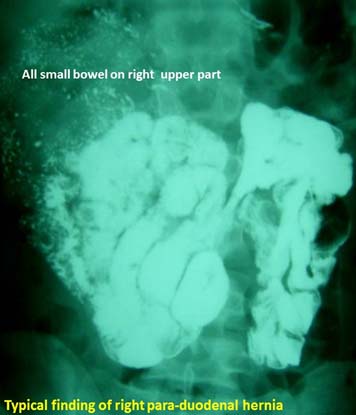
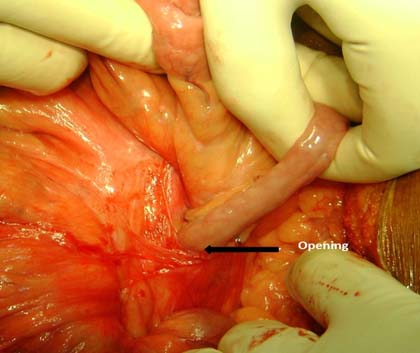
Often, they present at recurrent BO and keep resolving on conservative management. A CT scan with oral contrast and even a barium study of small bowel is usually diagnostic. Para-duodenal hernia constitute 50% of internal herniation [Table/Fig-7] [36,37]. A small bowel barium study/ CT-scan may be diagnostic [38]. Laparoscopy continues to be a safe diagnostic and therapeutic tool in the management of pediatric initial BO and recurrent small BO [39]. In case of any difficulty, an open surgical reduction [Table/Fig-8] can be undertaken.
Barium enema study showing all small bowel to right (right paraduodenal hernia).
Bowel coming out of internal hernia.
b. Early post-operative BO or early recurrence BO after relieve of BO/ paralytic ileus
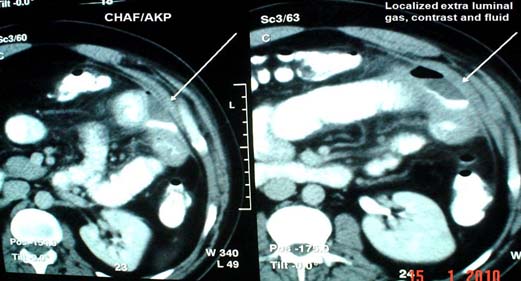
This is the most serious condition, as the patients has been fasting and still vomiting. This leads to malnutrition and electrolyte imbalance. When this state of post-operative ileus remains longer than usual, patient continues to have distended abdomen and continues to vomit. It is important to rule out any intestinal leak. It is mostly localized due to the adhesion [Table/Fig-9] and rarely leads to peritonitis. Any form of drainage will lead to a faecal fistula and, hence the critical decision can be taken on laparotomy and closure or create a fistula. Whatever may be the decision, parenteral nutrition must be instituted in this state of bowel failure. In case a stoma is created in jejunum or mid bowel, the distal bowel can be utilized in the way of distal feeding of the output [40]. This type of chyme re-infusion or enteroclysis are less expensive, well tolerated, and easy-to-use nutrition support techniques, which may allow reducing parenteral nutrition-related healthcare costs and lifesaving [40].
Localized leak as a cause of ileus.
The next step is to differentiate between prolonged paralytic ileus and mechanical BO. One should suspect mechanical obstruction when: Patient had passed flatus/stool and then it stopped again, noisy peristalsis and no flatus, colicky pain, X-ray/CT-scan/contrast study abdomen suggests obstruction [41]. The incidences of re-surgery are given in [Table/Fig-10]. Once it is established as mechanical obstruction and bowel is non gangrenous, a gastrograffin trail can be given and if it fails, then surgery can be done as per the algorithm.
Incidence of re-laparotomy in ileus like condition [41,43,44].
| S. No | Number of operation | Cases of relaparotomy | Relaparotomy due to ileus condition | % | Reference |
|---|
| 1 | 4908 | | 57 | 1.161 | [43] |
| 2 | 71588 | 1049 | 411 | 0.57 | [44] |
| 3 | 1795 | 30 | 24 | 1.331 | [41] |
Then only one can think of using alvimopan and methylnaltrexone, the peripherally acting μ-opioid receptor antagonists can be used to accelerate gastrointestinal recovery. In another randomized trial administration of Bisacodyl demonstrated significantly earlier bowel movements than those who received placebo (25 h vs. 56 h) [42]. Even in early post-operative adhesion the management protocol should follow the algorithm [Table/Fig-11].
c. Late post-operative recurrence
Recurrent BO is a real challenge in adhesive type. The common causes are given in [Table/Fig-6]. There are many more rare and uncommon causes besides given in the table. The initial management remains the same as applicable in the algorithms. In recurrent adhesive SBO, not resolving even after gastrograffin trail, surgery is indicated. During surgery, after adhesiolysis, it is better to do a plication of the bowel to avoid next attack [Table/Fig-12] [45]. The plication can be done by fixing the mesentery (The Noble plication) by Protective fibrin-sealed application [46]. Trans- mesenteric plication can also be done in a state of peritonitis [47]. But in the [Table/Fig-9] only sero-muscuarsuturings are used for plication to avoid mesenteric vascular accident. This patient has not had the 4th attack of BO after this plication. A long intra-luminal tube and or a long intestinal luminal tube [Table/Fig-13], as a stent may add the luminal patency even with serosal adhesion and can be given early feeding with multiple holes throughout the intra-luminal part of the tube and lower post-operative complications [48]. Studies are on to find an anti-adhesive intraperitoneal fluid or surface application like 4DryField(®) PH and Seprafilm(®). Only the surface application of 4DryField(®) PH and Seprafilm(®) showed significant adhesion prevention capabilities. 4DryField(®) PH achieved the highest adhesion prevention effectiveness without restrictions concerning mode of application and compatibility and, thus, is a promising strategy to prevent abdominal adhesions [49].
However, In a cochrane database systematic review, the positive results are refuted [50]. Similar, negative result is reported for icodextrin as an anti-adhesive intraperitoneal fluid [51]. In fact the cause of the recurrent obstruction must be found out and addressed once the acute obstruction resolves. Such cases are common in internal hernias. A barium study or CT scan with enteral contrast clinches the diagnosis. If both go without a diagnosis, a diagnostic laparoscopy will culminate to therapy as well.
If surgery is needed during the acute attack, laparotomy the not only gives the diagnosis but curative surgery can be done. The case of abdominal cocoon is challenging to the surgeon plain X-ray shows hardly any dilated small bowel to the inquisitive surgeon. At times it might be cocoon which covers the entire small bowel. It is mostly due to tuberculosis in India [52]. Occasionally it may be due to fibrosis of the peritoneum [53]. Tricky adhesiolysis by an experienced surgeon is needed.
Gastrograffin trial
Data showed that, the use of gastrograffin in adhesive small intestine obstruction is safe and reduces the operative rate and the time to resolution of obstruction. One study has reported the resolution of obstruction in 81.5% after a mean time of 6.4 hours [54]. When it resolves, it reduces the hospital stay with adhesive SBO [54–56] and failure to resolve is an effective indication in predicting the need for surgery [55]. It is better to do a CT scan with oral contrast to know the location and cause of obstruction so that it can be dealt with during this attack or be prepared in case of any recurrence.
Ovarian cancer related bowel obstruction
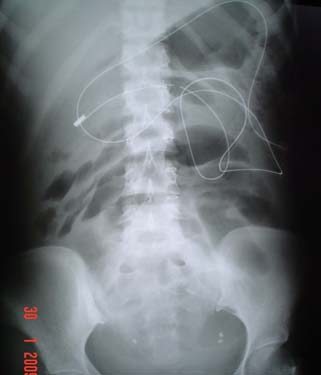
Ovarian cancer needs special mention due to the high incidence of BO in these patients. Nearly 20% of women developed BO after they are diagnosed with cancer. There is associated increased risk of subsequent obstructions [57]. Un-dilated bowel in presence of features of SBO does not benefit from operative intervention [Table/Fig-14]. Estimation of CA-125 may be helpful. Diligent discussion with the primary team and frank discussions with the patient and his or her family are essential to formulate an appropriate plan [58].
Undilated bowel in advanced ovarian cancer.
Timing and decision for surgery
Timing is crucial to avoid gangrenous bowel resection and obvious electrolyte imbalance. Identifying patients who may safely undergo non operative management remains difficult [59]. In a large study of 1613 patients 56.6% required surgery and 43.4% could be managed non-operatively [59]. There was an associated higher incidence of bowel resection in patients who took increased time to reach the operating room. Among the patients in whom the admission to operating room was less than 24 hours, 12% patients had bowel resection as compared to 29% in patients who took greater than 24 hours [59]. To avoid potentially increasing risk for bowel loss, intervention should be considered by the second day in a paediatric patient with low threshold in those who do not exhibit signs of improvement [31] and no more than 5 days in adults [60]. BO in young age and patients having virgin abdomen are more likely to under go operation [59]. Patients with a CT reading of complete obstruction, dilated small bowel and free fluid were operated on 77%, 66%, and 65% of the time, respectively [61].
Patients on conservative treatment for BO, where drainage volume through the nasogastric tube on day 3 is > 500mL, mostly required surgery [62]. CT scan of abdomen with oral gastrograffin not only gives the location of BO but also adds to the gastrograffin trial and avoids an abdominal surgery [63]. When a procedure is needed for adhesiolysis, laparoscopic adhesiolysis in expert hand in selected patients, reduced overall complication rate. It is found to be advantageous in studies [64]. The post-operative hospital stay was significantly shorter in the Laparoscopic Adhesiolysis (LA) group compared to converted (3 vs. 9 days). LA is safe and feasible for the management of BO and should be offered to all patients with BO unless there is an absolute contraindication to laparoscopic surgery [65,66]. It is also suitable in paediatric age group [67]. It is an excellent diagnostic tool and, in most cases, a therapeutic modality in patients with SBO. However, a significant number of patients will require conversion [66]. Open adhesiolysis is faster than laparoscopic adhesiolysis (LA) [68]. A previous upper abdominal surgical incision and a transition zone outside of the pelvis on CT scan were pre-operative predictors of a successful laparoscopic adhesiolysis. The laparoscopic group had shorter length of stay [69]. LA in presence of dilated bowel leads to less dolmen for the play of the instruments and hence, it is not advisable. It is ideal, where BO caused by post-operative adhesion had resolved earlier on conservative management and the patient comes with recurrence, where LA to be attempted early before gross dilatation of the bowel [70]. Conversion to mini-laparotomy or laparotomy should be considered in patients with dense or pelvic adhesion [71].
Once the diagnosis of BO is established clinically and confirmed by radiological investigation; then comes the decision. There are two decisions conservative or operative. If conservative is chosen, the responsibility of the treating team is to operate before the bowel becomes gangrenous. Hence, repeating X-ray/CT scan and radiating the patient looks non-academic. Secondly clinical diagnosis of resolution of BO is quite evident clinically.
Palliation in BO
In palliative care patients with nausea and vomiting, 5HT3 receptor antagonists can be used if treatment with other antiemetics, such as metoclopramide and neuroleptics is not sufficient.
There is a trend that steroids in combination with other antiemetic improve symptom relief.
Cannabinoids have a status as a second line antiemetic. As a palliative care in malignant obstruction, long acting octreotide remains the first choice and butyl-scopolammonium bromide, the second to palliate the symptoms [72]. In a small study of only 12 cases, (patients with malignant BO/ bowel dysfunction), most of the patients improved with combination of anti-inflammatory (Dexamethasone), anti-secretory (Octriotide), and prokinetic (metoclopramide).
Post-operative prognosis
The early post-operative mortality is strongly linked with the age and the American Society of Anesthesiologists (ASA) grade and the long-term mortality with post-operative complications [73]. More frequent bowel resections might be suggested for patients featuring 10 or more obstructive strictures and an intestinal wall injury, especially when associated with a reversible intestinal ischemia [74].
Conclusion
Predicting the conservative or operative management in BO is difficult. Decision on surgery should be taken in paediatric patient by 24 hours, in young age, in virgin abdomen and large BO by 48hrs and within 3-5 days of admission in adults, if the oral gastrografin fails to resolve the BO more so the adhesive obstruction with high (>500mL) gastric tube aspirate (Algorithm). In recurrent BO some form of plication may be considered during surgery. The early post-operative mortality is strongly linked with the age and the ASA grade whereas the long-term mortality is associated with post-operative complications.
[1]. Drozdd W, Budzynski P, Change in mechanical bowel obstruction demographic and aetiological patterns during the past century: observations from one health care institutionArch Surg 2012 147:175-80. [Google Scholar]
[2]. Cirocchi R, Abraha I, Farinella E, Montedori A, Sciannameo F, Laparoscopic versus open surgery in small bowel obstructionCochrane Database Syst Rev 2010 17(2):CD007511 [Google Scholar]
[3]. Miller G, Boman J, Shrier I, Gordon PH, Natural history of patients with adhesive small bowel obstructionBr J Surg 2000 87:1240-47.PMID: 10971435 [Google Scholar]
[4]. Lo OS, Law WL, Choi HK, Lee YM, Ho JW, Seto CL, Early outcomes of surgery for small bowel obstruction: analysis of risk factorsLangenbecks Arch Surg 2007 392:173-78. [Google Scholar]
[5]. Zielinski MD, Eiken PW, Bannon MP, Heller SF, Lohse CM, Huebner M, Sarr MG, Small bowel obstruction-who needs an operation? A multivariate prediction modelWorld J Surg 2010 34:910-19. [Google Scholar]
[6]. Loughborough W, Development of a plain radiograph requesting algorithm for patients presenting with acute abdominal painQuant Imaging Med Surg 2012 2:239-44. [Google Scholar]
[7]. Musson RE, Bickle I, Vijay RK, Gas patterns on plain abdominal radiographs: a pictorial reviewPostgrad Med J 2011 87:274-87. [Google Scholar]
[8]. Qalbani A, Paushter D, Dachman AH, Multidetector row CT of small bowel obstructionRadiol Clin North Am 2007 45:499-512. [Google Scholar]
[9]. Desser TS, Gross M, Multidetector row computed tomography of small bowel obstructionSemin Ultrasound CT MR 2008 29:308-21. [Google Scholar]
[10]. Shah ZK, Uppot RN, Wargo JA, Hahn PF, Sahani DV, Small bowel obstruction: the value of coronal reformatted images from 16-multidetector computed tomography a clinicoradiological perspectiveJ Comput Assist Tomogr 2008 32:23-31. [Google Scholar]
[11]. Colon MJ, Telem DA, Wong D, Divino CM, The relevance of transition zones on computed tomography in the management of small bowel obstructionSurgery 2010 147:373-77. [Google Scholar]
[12]. Atamanalp SS, Sigmoid volvulus: diagnosis in 938 patients over 45.5 yearsTech Coloproctol 2013 17:419-24. [Google Scholar]
[13]. Oren D, Atamanalp SS, Aydinli B, Yildirgan MI, Basoglu M, Polat KY, An algorithm for the management of sigmoid colon volvulus and the safety of primary resection: experience with 827 casesDis Colon Rectum 2007 50:489-97. [Google Scholar]
[14]. Osiro SB, Cunningham D, Shoja MM, Tubbs RS, Gielecki J, Loukas M, The twisted colon: a review of sigmoid volvulusAm Surg 2012 78:271-79. [Google Scholar]
[15]. McCullough JA, Engledow AH, Treatment options in obstructed left-sided colonic cancerClin Oncol (R Coll Radiol) 2010 22:764-70. [Google Scholar]
[16]. Calleja JL, Delgado S, del Val A, Hervás A, Larraona JL, Terán Á, Ferric carboxymaltose reduces transfusions and hospital stay in patients with colon cancer and anaemiaInt J Colorectal Dis 2016 31:543-51. [Google Scholar]
[17]. An MS, Yoo JH, Kim KH, Bae KB, Choi CS, Hwang JW, T4 stage and preoperative anaemia as prognostic factors for the patients with colon cancer treated with adjuvant FOLFOX chaemotherapyWorld J Surg Oncol 2015 13:64 [Google Scholar]
[18]. Blake P, Delicata R, Cross N, Sturgeon G, Hargest R, Large bowel obstruction due to colorectal carcinoma can be safely treated by colonic stent insertion—case series from a UK district general hospitalColorectal Dis 2012 14:1489-92. [Google Scholar]
[19]. White SI, Abdool SI, Frenkiel B, Braun WV, Management of malignant left-sided large bowel obstruction: a comparison between colonic stents and surgeryANZ J Surg 2011 81:257-60. [Google Scholar]
[20]. Zhang Y, Shi J, Shi B, Song CY, Xie WF, Chen YX, Self-expanding metallic stent as a bridge to surgery versus emergency surgery for obstructive colorectal cancer: a meta-analysisSurg Endosc 2012 26:110-19. [Google Scholar]
[21]. Cirocchi R, Farinella E, Trastulli S, Desiderio J, Listorti C, Boselli C, Safety and efficacy of endoscopic colonic stenting as a bridge to surgery in the management of intestinal obstruction due to left colon and rectal cancer: a systematic review and meta-analysisSurg Oncol 2013 22:14-21. [Google Scholar]
[22]. Gorissen KJ, Tuynman JB, Fryer E, Wang L, Uberoi R, Jones OM, Local recurrence after stenting for obstructing left-sided colonic cancerBr J Surg 2013 100:1805-09. [Google Scholar]
[23]. Zaharie F, Mocan L, Mocan T, Tomus C, Hodor V, Al Hajjar, Surgical management of malignant large bowel obstructionsChirurgia (Bucur) 2011 106:479-84. [Google Scholar]
[24]. Tohmé C, Chakhtoura G, Abboud B, Noun R, Sarkis R, Ingea H, Subtotal or total colectomy as surgical treatment of left-sided occlusive colon cancerJ Med Liban 2008 56:198-202. [Google Scholar]
[25]. Käser SA, Glauser PM, Künzli B, Dolanc R, Bassotti G, Maurer CA, Subtotal colectomy for malignant left-sided colon obstruction is associated with a lower anastomotic leak rate than segmental colectomyAnticancer Res 2012 32:3501-05. [Google Scholar]
[26]. Shimura T, Joh T, Evidence-based Clinical Management of Acute Malignant Colorectal ObstructionJournal of Clin Gastroenterol 2016 50:273-85. [Google Scholar]
[27]. Gash K, Chambers W, Ghosh A, Dixon AR, The role of laparoscopic surgery for the management of acute large bowel obstructionColorectal Dis 2011 13:263-66. [Google Scholar]
[28]. Frago R, Ramirez E, Millan M, Kreisler E, del Valle E, Biondo S, Current management of acute malignant large bowel obstruction: a systematic reviewAm J Surg 2014 207:127-38. [Google Scholar]
[29]. Juang D, Snyder CL, Neonatal bowel obstructionSurg Clin North Am 2012 92:685-711. [Google Scholar]
[30]. Mohiuddin SS, Gonzalez A, Corpron C, Meckel’s diverticulum with small bowel obstruction presenting as appendicitis in a pediatric patientJSLS 2011 15:558-61. [Google Scholar]
[31]. Lautz TB, Raval MV, Reynolds M, Barsness KA, Adhesive small bowel obstruction in children and adolescents: operative utilization and factors associated with bowel lossJ Am Coll Surg 2011 212:855-61. [Google Scholar]
[32]. Sule AZ, Bada D, Nnamonu MI, Postoperative non-adhesive mechanical intestinal obstruction: a review of seven casesNiger J Med 2009 18:63-7.PMID:19485151 [Google Scholar]
[33]. Duron JJ, Silva NJ, du Montcel ST, Berger A, Muscari F, Hennet H, Adhesive postoperative small bowel obstruction: incidence and risk factors of recurrence after surgical treatment: a multicenter prospective studyAnn Surg 2006 244:750-57. [Google Scholar]
[34]. Farid S, Iqbal A, Gechev Z, Re: Adhesive intestinal obstruction In laparoscopic versus open colorectal resectionColorectal Dis 2012 :15 [Google Scholar]
[35]. Williams SB, Greenspon J, Young HA, Orkin BA, Small bowel obstruction: conservative vs. surgical managementDis Colon Rectum 2005 48:1140-46. [Google Scholar]
[36]. Moran JM, Salas J, Sanjuan S, Amaya JL, Rincon P, Serrano A, Paramesocolic hernias: consequences of delayed diagnosis. Report of three new casesJ Pediatr Surg 2004 39:112-16. [Google Scholar]
[37]. Brigham RA, Paraduodenal hernia. In: LM Nyhus, RE Condon. 50(ed.)Hernia 1995 4th edPhiladelphiaLippincott Williams and Wilkins [Google Scholar]
[38]. Pujahari AK, Rohit M, Two cases of Right para-duodenal hernia and review of literatureGastroenterology Report 2014 :1-4. [Google Scholar]
[39]. Alemayehu H, David B, Desai AA, Iqbal CW, St Peter SD, Laparoscopy for small bowel obstruction in children—an updateJ Laparoendosc Adv Surg Tech A 2015 25:73-6. [Google Scholar]
[40]. Thibault R, Picot D, Chyme reinfusion or enteroclysis in nutrition of patients with temporary double enterostomy or enterocutaneous fistulaCurr Opin Clin Nutr Metab Care 2016 [Epub ahead of print] [Google Scholar]
[41]. Pujahari AK, (chapter) postoperative ileus in Spinger publication Edtr TK Chottopadhya’s GI Surgery annual 1999 6:26-33. [Google Scholar]
[42]. Zeinali F, Stulberg JJ, Conor P, Delaney, Pharmacological management of postoperative ileusCan J Surg 2009 52:153-57. [Google Scholar]
[43]. Krasil’nikov DM, Skobelkin OK, Fèdorov VV, Tverskov SV, Zaripov NZ, [Early postoperative adhesion ileus]Vestn Khir Im II Grek 1994 152:17-21. [Google Scholar]
[44]. Bondarenko IN, Relaparotomy in the surgical treatment of early acute postoperative ileusKlin Khir 1997 (7-8):19-21. [Google Scholar]
[45]. Dinstl K, Lechner G, Riedl P, Schiessel R, [Late results of The Noble operation. Clinical and radiological follow-up examination]MMW Munch Med Wochenschr 1976 118:29-30. [Google Scholar]
[46]. Holland-Cunz S, Boelter AV, Waag KL, Protective fibrin-sealed plication of the small bowel in recurrent laparotomyPediatr Surg Int 2003 19:540-43. [Google Scholar]
[47]. Sclabas G, Heller G, Lüdin A, Odstrcilik E, Ammann J, Late results of Childs-Phillips mesenteric plication for therapy and prevention of small intestine ileusChirurg 1997 68:693-99. [Google Scholar]
[48]. Li M, Ren J, Zhu W, Li Y, Zhao Y, Jiang J, Retrograde long intestinal tube splinting: a safe and effective treatment for preventing recurrent adhesive small bowel obstructionHepatogastroenterology 2014 61:1287-91. [Google Scholar]
[49]. Poehnert D, Grethe L, Maegel L, Jonigk D, Lippmann T, Kaltenborn A, Evaluation of the Effectiveness of Peritoneal Adhesion Prevention Devices in a Rat ModelInt J Med Sci 2016 13:524-32.eCollection 2016 [Google Scholar]
[50]. Hindocha A, Beere L, Dias S, Watson A, Ahmad G, Adhesion prevention agents for gynaecological surgery: an overview of Cochrane reviewsCochrane Database Syst Rev 2015 [Google Scholar]
[51]. Sakari T, Sjödahl R, Påhlman L, Karlbom U, Role of icodextrin in the prevention of small bowel obstruction. Safety randomized patients control of the first 300 in the ADEPT trialColorectal Dis 2016 18:295-300. [Google Scholar]
[52]. Anantha RV, Salvadori MI, Hussein MH, Merritt N, Abdominal cocoon syndrome caused by Mycobacterium bovis from consumption of unpasteurised cow’s milkLancet Infect Dis 2015 15:1498 [Google Scholar]
[53]. Abid S, Arisar FA, Memon WA, Abdominal Cocoon: Primary Sclerosing Encapsulating PeritonitisAm J Gastroenterol 2016 111:923doi: 10.1038/ajg.2016.72 [Google Scholar]
[54]. Di Saverio S, Catena F, Ansaloni L, Gavioli M, Valentino M, Pinna AD, Water-soluble contrast medium (gastrografin) value in adhesive small intestine obstruction (ASIO): aprospective, randomized, controlled, clinical trialWorld J Surg 2008 32:2293-304. [Google Scholar]
[55]. Branco BC, Barmparas G, Schnüriger B, Inaba K, Chan LS, Demetriades D, Systematic review and meta-analysis of the diagnostic and therapeutic role of water-soluble contrast agent in adhesive small bowel obstructionBr J Surg 2010 97:470-78. [Google Scholar]
[56]. Abbas S, Bissett IP, Parry BR, Oral water soluble contrast for the management of adhesive small bowel obstructionCochrane Database Syst Rev 2007 18:CD004651 [Google Scholar]
[57]. Mooney SJ, Winner M, Hershman DL, Wright JD, Feingold DL, Allendorf JD, Bowel obstruction in elderly ovarian cancer patients: a population-based studyGynecol Oncol 2013 129:107-12. [Google Scholar]
[58]. DeBernardo R, Surgical management of malignant bowel obstruction: strategies toward palliation of patients with advanced cancerCurr Oncol Rep 2009 11:287-92. [Google Scholar]
[59]. Leung AM, Vu H, Factors predicting need for and delay in surgery in small bowel obstructionAm Surg 2012 78:403-07. [Google Scholar]
[60]. Schraufnagel D, Rajaee S, Millham FH, How many sunsets? Timing of surgery in adhesive small bowel obstruction: a study of the nationwide inpatient sampleJ Trauma Acute Care Surg 2013 74:181-87.discussion 187-9 [Google Scholar]
[61]. Jones K, Mangram AJ, Lebron RA, Nadalo L, Dunn E, Can a computed tomography scoring system predict the need for surgery in small-bowel obstruction?Am J Surg 2007 194:780-83.discussion 783-4 [Google Scholar]
[62]. Sakakibara T, Harada A, Yaguchi T, Koike M, Fujiwara M, Kodera Y, The indicator for surgery in adhesive small bowel obstruction patient managed with long tubeHepatogastroenterology 2007 54:787-90. [Google Scholar]
[63]. Wilson MS, Ellis H, Menzies D, Moran BJ, Parker MC, Thompson JN, A review of the management of small bowel obstruction. Members of the Surgical and Clinical Adhesions Research Study (SCAR)Ann R Coll Surg Engl 1999 81:320-28. [Google Scholar]
[64]. Li MZ, Lian L, Xiao LB, Wu WH, He YL, Song XM, Laparoscopic versus open adhesiolysis in patients with adhesive small bowel obstruction: a systematic review and meta-analysisAm J Surg 2012 204:779-86. [Google Scholar]
[65]. Qureshi I, Awad ZT, Predictors of failure of the laparoscopic approach for the management of small bowel obstructionAm Surg 2010 76:947-50. [Google Scholar]
[66]. Kirshtein B, Roy-Shapira A, Lantsberg L, Avinoach E, Mizrahi S, Laparoscopic management of acute small bowel obstructionSurg Endosc 2005 19:464-67. [Google Scholar]
[67]. Lee J, Tashjian DB, Moriarty KP, Surgical management of pediatric adhesive bowel obstructionJ Laparoendosc Adv Surg Tech A 2012 22:917-20. [Google Scholar]
[68]. Okamoto H, Wakana H, Kawashima K, Fukasawa T, Fujii H, Clinical outcomes of laparoscopic adhesiolysis for mechanical small bowel obstructionAsian J Endosc Surg 2012 5:53-8. [Google Scholar]
[69]. Simmons JD, Rogers EA, Porter JM, Ahmed N, The role of laparoscopy in small bowel obstruction after previous laparotomy for trauma: an initial reportAm Surg 2011 77:185-87. [Google Scholar]
[70]. Wang Q, Hu ZQ, Wang WJ, Zhang J, Wang Y, Ruan CP, Laparoscopic management of recurrent adhesive small-bowel obstruction: Long-term follow-upSurg Today 2009 39:493-99. [Google Scholar]
[71]. Tsumura H, Ichikawa T, Murakami Y, Sueda T, Laparoscopic adhesiolysis for recurrent postoperative small bowel obstructionHepatogastroenterology 2004 51:1058-61. [Google Scholar]
[72]. Benze G, Geyer A, Alt-Epping B, Nauck F, Treatment of nausea and vomiting with 5HT3 receptor antagonists, steroids, antihistamines, anticholinergics, somatostatinantagonists, benzodiazepines and cannabinoids in palliative care patients: a systematic reviewSchmerz 2012 26:481-99. [Google Scholar]
[73]. Jeffrey B, Paula L, Lucan R, Medical Therapy of malignant bowel obstruction with Octreotide, Dexamethasone, and MetoclopramideAm J Hosp & Palliat care 2016 33:407-10. [Google Scholar]
[74]. Duron JJ, du Montcel ST, Berger A, Muscari F, Hennet H, Veyrieres M, French federation for surgical research. Prevalence and risk factors of mortality and morbidity after operation for adhesive post-operative small bowel obstructionAm J Surg 2008 195:726-34. [Google Scholar]