Antibodies to hepatitis B surface antigens (Anti-HBs) are important markers of immunity against Hepatitis B. The detection of this antibody titer is essential in evaluating the vaccine response. Antibody titer of >10 mIU/mL after one to two months of completing primary vaccination schedule is considered seroprotective [1]. Over time and age the antibody titers are known to decrease. It has also been observed that individuals with some detectable antibody levels are likely to respond better to booster dose [2]. Therefore measuring the titer plays a major role in decision making for post-exposure prophylaxis. Enzyme Linked Immunosorbent Assay (ELISA) and Chemiluminescence Immunoassay (CLIA) are two frequently employed tests for quantification of Anti-HBs, among other tests, including Radioimmunoassay and micro-particle enzyme immunoassay (MEIA) [3]. However, ELISA and CLIA are based on different test-principles. Quantitative ELISA is a direct, antibody sandwich enzyme assay which utilizes native HBsAg (Subtypes ad and ay) as solid phase and also horseradish peroxidase labelled HBsAg as conjugate, with the underlying principle being a calorimetric method. Method utilizes the intensity of color produced by reaction between conjugate and substrate to measure the amount of antibodies present. CLIA on the other hand, utilizes recombinant HBsAg coated paramagnetic micro particles that bind to Anti-HbsAg in serum and acridinium labelled rHBsAg coated particles as conjugates. Antibody concentration is determined by the light emitted on antigen-antibody reaction and measured using Relative Light Units (RLU). Though studies have evaluated and compared test performance based on platforms which work on same principles [4,5] there is paucity of analysis between two different test formats. The objective of this study was to analyse the degree of agreement between ELISA and CLIA in identifying protective and non-protective titers and variation in the measurement of Anti-HBs levels between the two methods.
Materials and Methods
In this prospective comparative study, consecutive serum samples sent to the laboratory for Anti-HBs quantification between May and June 2016 were subjected to measurement by both ELISA (Bio-Rad laboratories, Redmond WA) and CLIA (Abbott, Chicago, IL), after obtaining approval from institutional ethics committee. Haemolyzed samples and lipaemic samples were excluded from the study.
Both the test methods were performed as per the manufacturer’s guidelines [6,7]. The standard curve in ELISA was obtained using five calibrators with known antibody concentrations of 0, 10, 100, 400 and 1000mIU/mL [7]. The values of unknown samples were obtained using standard curve. In CLIA, the serum antibody levels is determined using a previously generated Anti-HBsAg calibration curve, with six calibrators of known antibody concentration of 0, 10, 50, 100, 500 and 1000mIU/mL [6].
Antibody titers ≤10mIU/mL were considered non-protective and >10 mIU/mL as protective. Discrepant value between the two tests was defined as values differing by >2 multiplication factor [5] with lower value among each set of values as denominator.
Statistical Analysis
Statistical analysis was performed using SPSS (version 15). The interpretative outcome of both the tests was evaluated for the degree of agreement using kappa coefficient. Individual values given by the test methods were analysed for discrepant values. Bland – Altman analysis was used to compare the assays with each other.
Results
A total of 69 serum samples were sent for quantification of Anti-HBs during the study period and were subjected CLIA in addition to the ELISA, which is the test used in department for Anti-Hbs quantification. Eighteen samples (26.1%) were of health care personnel, and the remaining 51 (73.9%) were of patients visiting the hospital. Seventeen samples (24.6%) were of end-stage renal disease patients on haemodialysis, and the remaining samples were of patients needing Anti-HBs quantification for other reasons.
Comparison of interpretative results
Of the 69 samples tested, ELISA identified 58 samples (84.1%) as having protective antibody titers and 11 (15.9 %) as non-protective. By CLIA, 12 samples (17.4 %) were identified as non-protective and 57 (82.6%) as protective. The two tests disagreed on three samples (4.3%); two samples deemed protective by ELISA were reported as non-protective by CLIA. One non-protective titer by ELISA was reported as protective by CLIA.
On evaluation of assay interpretation, percentage that were identified as protective and non-protective by evaluating the titers by the both the assays were 96.5% and 90.9% respectively, resulting in an agreement of κ = 0.84.
Comparison of titers measured by two assay methods
Mean titer values by ELISA and CLIA were 503mIU/mL and 332mIU/mL, respectively. When values were categorized, Chemiluminescence reported majority of titers in <400mIU/mL range (51 samples; 73.9%) compared to ELISA (36 samples; 52.2%) which more frequently reported values > 400mIU/mL [Table/Fig-1].
Graph comparing the antibody titers reported by the two tests.
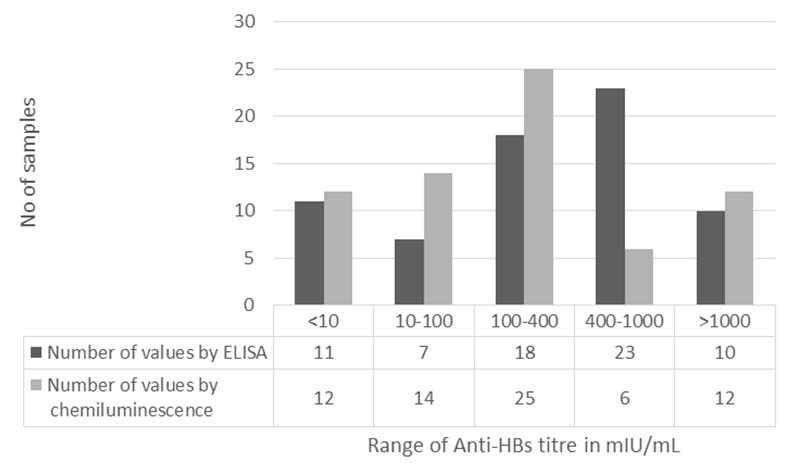
Overall coefficient of variation (CV) of titers by ELISA was 74.5% and titers by CLIA were 113.1%. Factor of multiplication between individual sets of values ranged from 0.2 to 28. When the titer values > 10 mIU/mL were analysed, there were 32 discrepant values which showed difference of >2 multiplication factor. Among the discrepant values in 28 samples ELISA value was higher than CLIA and in rest of 4 samples CLIA titer was more than ELISA. Difference in CV of discrepant values by both methods was more than 10% whereas it was <10% and acceptable in case of non-discrepant results [Table/Fig-2].
Extent of difference between ELISA and Chemiluminescence among discrepant and non-discrepant titers.
| Mean value in mIU/mL (SD) | Coefficient of Variation (CV) | Difference between CV |
|---|
| Discrepant value | CLIA | 166.7 (178.0) | 106.7% | 42% |
| ELISA | 545.6 (353.3) | 64.7% |
| Discrepant value: (Difference between two values) | ELISA >CLIA | 460.4 (262.6) | 56.9% | 37.6% |
| CLIA >ELISA | 192.2 (181.6) | 94.5% |
| Non-discrepant value | ELISA | 647.5 (381.5) | 58.9% | 8.9% |
| CLIA | 637.4 (380.0) | 50.0% |
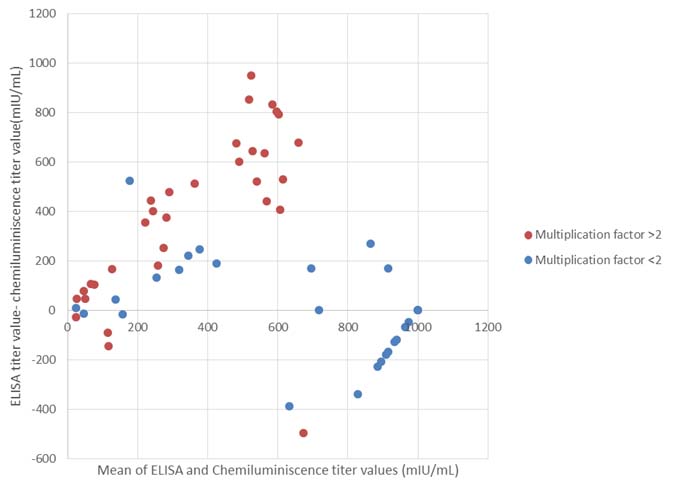
A Bland-Altman plot was done for the two groups which showed a linearly increasing difference in both sets of values with a range upto 1000mIU/mL in case of discrepant and 400mIU/mL in case of non-discrepant values [Table/Fig-3].
Bland Altman plot comparing the discrepant (multiplication factor >2, in red) and non-discrepant (multiplication factor <2, in blue) results of ELISA and CLIA.
Both ELISA and CLIA work on different assay formats and also differ in assay characteristics [Table/Fig-4].
Comparison of assay characteristics.
| Assay characteristics | ELISA | CLIA |
|---|
| Minimum Sample required | 75μl | 150μl |
| Number of controls/Standards | 5 | 5 |
| Time to results | 180 minutes | 30 minutes |
| Assay platform | Automated / Manual | Automated |
| Technical expertise | High if manual | Low |
Discussion
Antibodies to Hepatitis B surface antigen is usually measured to identify if a person developed adequate immune-response following vaccination, whether a health-care professional has protective titers, and in post-exposure management decisions [8]. While ELISA is the commonly employed test for Anti-HBs measurement at our center, CLIA offers the advantage of rapid turn-over and is less demanding in technical expertise. In this study, we compared the two assays for their concordance in reporting Anti-HBs levels. From clinical end-point, interpretation of the titer value is more important than the absolute value itself. In terms of classifying a serum antibody level as protective or not (based on measured titers being > or ≤10 mIU/mL, respectively), there was a high degree of agreement between ELISA and CLIA, with a kappa agreement of 0.84. The two tests disagreed on three samples; two samples reported as protective by ELISA were categorized as non-protective, and one sample which was non-protective by ELISA was categorized as protective by CLIA. CLIA has been shown to compare favorably with ELISA in antibody detection for several viral infections including measles and rubella [9]. Both tests have also been similarly effective in HBs antigen detection [10]. However, very few papers have reported on comparison between the two in quantifying Anti-HBs [5].
While classifying a serum titer as protective or non-protective is the principal application of antibody-quantifying test, absolute titers <100mIU/mL raise clinical dilemmas about effectiveness of vaccine and need for post-exposure prophylaxis [8]. Therefore, we compared the absolute titer values to evaluate the discrepancy reported by the two tests. Despite a high degree of agreement between the two tests when they were categorized, there was a substantial variation in the absolute titers between them. This pattern prevailed even in the three samples where the tests disagreed. For the two samples that were ELISA positive but CLIA negative, the factor of multiplication were 11.3 and 13 respectively. For the sample that was considered non-protective by ELISA but protective by CLIA, it was 0.2. Nearly half of the tested samples in our study had titers reported by the tests differing by greater than two factor of multiplication. The reason for such a high variation is difficult to explain. This could have been clarified by repeating the test, but due to limited availability of kits, we did not check for reproducibility. The anti-HBs measurement, like HBs antigen quantification tests [11], is known to vary between different measurement kits, sometimes even within the same platform. Results reported by Oone et al., in a study evaluating Lumipulse G1200 and Abbott i2000 tests for anti HBs antibodies titers [4] has shown lower antibody titre by Abbott i2000 as compared to Lumipulse G1200. Similarly in another study comparing two CLIA based kits, the concordance was reported to be low, especially so in samples with values <50mIU/mL [3]. However, the variation could also have been due to other reasons. As an example, ‘escape mutants’ of the virus have resulted in infections without detectable anti-HBs antibodies [12]. In another similar study, Huzly et al., compared nine immunoassays for anti-HBs antibody measurement wherein they had 18 highly discrepant sample values out of a total of 200 samples. They also have reported a bigger range of factor of multiplication (2.8 -105) [5]. According to their study this discrepancy was observed more in a group of vaccinated healthy individuals. This may also have been due to more number of assays used in that study. In addition to difference in vaccine antigens, antigens used in various assays, antibodies with low avidity, difference in subclasses of IgG, endogenous proteins in the sample etc., are also known to affect the immunoassay [5]. Similarly, low albumin levels in patients on haemodialysis have also been known to affect the measured antibody titers [13]. Thus, other factors could also have contributed to the discrepancies between the tests in our study. In general, reporting a protective titer as non-protective is less harmful than reporting a non-protective titer as protective as it may only result in an extra dose of vaccination [5].
Even though CLIA generally reported lower titers, CV in the measured titers was higher in CLIA than in ELISA, suggesting a wider range of reported values in CLIA. Among the discrepant samples, the CV between the two was even greater, indicating a higher degree of discrepancy. At higher Anti-HBs titers, the discrepancy between CLIA and ELISA appeared to reduce. Results of both the assays appeared to match when they were near the lower and upper linearity limits of the standards used in both the assays. This variation in inter-test performance over a range of measured antibody concentrations is known to occur. Other investigators have also reported that substantial difference exists between the titers obtained by various types of immunoassays, but with a good concordance value [3,14]. Likewise, though there is no similar study on Anti HBs, at least one report on HBs antigen detection suggests that at very low concentrations (<1 ng/mL), CLIA is superior to ELISA in determination of the antigen [15], despite equivalent performance of the two at higher antigen concentrations.
Most of the previous studies on comparison of two quantitative immunoassays have used correlation and regression studies. But this method evaluates the relationship between two quantitative variables and does not compare them. It has been suggested in many articles that the use of correlation and regression studies may not be ideal for comparing two values which measure same biological indicator [16,17]. The alternative method is by plotting a Bland-Altman analysis. This method plots the difference between two paired measurements against their mean which in turn evaluates the measure of agreement between the values. The plot shows a wider variation in values with higher discrepancies with an order up to 1000mIU/mL. As mentioned above, disparity between the outcomes of immunoassays is well documented. This variation may be due to difference in the calibrators used. The type of vaccine and the reagents used for testing have also been shown to influence the measured anti-HBs antibody levels [18].
In addition to comparing the test results it is also important to understand the advantages and disadvantages of both the assays. Though it showed a greater inter-sample variation (despite reporting lower titres) in our study, considering the benefits of ease of performance and rapid turn-over time while maintaining a high concordance with ELISA make CLIA an attractive choice for routine quantification of Anti-HBs. The single set of calibrators for the lot also makes it less error prone. On the other hand, ELISA would be more time consuming and also test-to-test calibration may increase chances of error, but it offers the advantage of being less dependent on specialized equipment.
Limitation
Our study is limited by a small number of sera testing non-protective, consequent to a small sample size. It also didn’t address the issues of accuracy and reproducibility of the individual tests due to the limited numbers of available CLIA kits. Also, the study doesn’t have the ability to pick a superior test or to state the sensitivity/specificity, because additional tests would be required to identify the accuracy and reproducibility of the tests. Additionally, variation within the test has not been accounted for. However, in terms of clinical applicability, both tests appear to be equally good in recognizing a non-protective antibody titer, thus shifting the decision to choose the optimum test on other factors, including cost, time and availability of technical expertise.
Conclusion
Both ELISA and CLIA were equally competent in identifying protective and non-protective antibody titer against Hepatitis B, with a kappa coefficient of 0.84 indicating an almost perfect agreement. However, in terms of absolute titer values, there was a substantial difference between the two, with CLIA in general reporting lower antibody titers when compared to ELISA. This may be due to variation in the standard calibrators used in each assay. Though CLIA showed more variation in the values, it has the advantage of being automated test with low turnaround time. Therefore both the test methodologies can be reliably used in place of each other for detection of Anti- HBs titer.