Breast Cancer (BC) is a common disease worldwide. In egypt, BC is ranked the first of female cancers with an incidence of 32.04% [1]. BC is a heterogeneous disease with distinctive clinical presentation, pathologic, biologic features and behaviour as well. Over the last 15 years, gene expression profiling characterized 4 major groups of BC, which classified patients into Luminal A, Luminal B, HER-2/neu enriched, and Triple Negative BC (TNBC); based on immunohistochemical staining for Estrogen Receptor (ER), Progesterone Receptor (PR), HER-2/neu and Ki-67 staining [2]. This helped stratification of BC patients for prognostic and therapeutic purposes.
Androgen Receptor (AR) belongs to steroid nuclear receptor family, it is a member of the nuclear superfamily involved in complex signaling network that plays role in cell proliferation [3]. It was found to play a role in tumourigenesis of cancers in prostate [4] and breast [5]. It was also found to be associated with good prognosis and to be related to ER and PR expression in BC [6,7], but AR expression in relation to different molecular carcinoma subtypes is less clearly understood.
In the current study, the immunohistochemical expression of AR was evaluated in 81 cases of BC from egyptian patients. Its expression was correlated with the standard clinico-pathologic variables, molecular subtype of BC and the Overall Survival (OS) of BC cases.
Materials and Methods
Case Selection
This retrospective study was conducted on 81 archival cases of Egyptian BC patients, which were diagnosed and retrieved from archives of pathology department, faculty of medicine, menoufia university, egypt spanning the period between 2008-2011. Included cases were mastectomy specimens from patients who haven’t received prior chemotherapy, with available panel of ER, PR, HER-2/neu and Ki-67 slides. Clinical data were retrieved from patient’s medical records including patient’s age, menopausal status and the OS. Cases were staged following the updated (7th) edition of the american joint committee on cancer (AJCC), cancer staging manual [8]. The Nottingham Prognostic Index (NPI) was calculated and categorized into good, moderate and poor [9].
Morphologic Evaluation
Haematoxylin & Eosin (H&E) stained sections were microscopically evaluated, based on the 2012 WHO classification of the tumours of breast [10]. Tumour size, histologic type, presence of in-situ component, Lymphovascular Invasion (LVI) and nodal status were evaluated. The cases were graded according to the modified bloom- richardson scheme [11].
Immunohistochemistry (IHC) Staining Procedure
IHC staining of AR was performed on 4μm-thickness sections. Microwave antigen retrieval (20 min; 10mmol/citrate buffer, pH 6.0) was done followed by inhibition of endogenous peroxidase activity (hydrogen peroxidase for 15 min). The primary antibody used was a mouse monoclonal antibody against AR (Clone AR441) at 1:50 dilution (Code MS-443P0). Thermo Fisher Scientific Anatomical Pathology (Fremont, CA)] was applied to the slides, the detection of bound antibody was accomplished using a modified labelled avidin-biotin reagent for 20 minutes. Slides were counterstained with Mayer’s haematoxylin for 5 minutes. Positive control (normal human prostate) and negative control (step of primary antibody omitted) were included with each staining run.
Immunohistochemical Stains Evaluation & Molecular Subtying
The slides and IHC stains were reviewed by 2 pathologists, blinded to the patient’s clinical data and a consensus agreement was obtained for the scores. The following cut-off levels were used for assessment of the IHC-stained slides. For ER and PR, the case was considered positive if ≥1% of the tumour nuclei were immunoreactive regardless of the intensity of staining [12]. HER-2/neu was considered positive only in 3+ staining intensity which was given to cases with >10% intense complete membrane staining [13]. The Ki-67 Labeling Index (Ki-67 LI) was determined semi-quantitatively. Positive nuclear speckled or granular stained cells were counted. A cut-off point of 14% for the cells that were labelled Ki-67 positive was used to categorize cases into either low proliferative or high proliferative [2]. A panel of 4 markers (ER, PR, HER-2/neu, and Ki-67) was used to determine molecular subtype of BC according to st. gallen international expert Consensus [2]. AR was positive when more than 1% of tumour cells showed nuclear immunoreactivity [14].
Statistical Analysis
Data were collected, tabulated and statistically analysed using software package for statistical analysis (SPSS, Version 22.0). Chi-square test was used to analyse the association between AR expression and cinico-pathologic variables. Survival data were evaluated using the Kaplan-Meier analysis. Statistical significance was established when p-value is ≤0.05.
Results
Patient Characteristics
A total 81 cases of BC (age range 30-81 years), were included in this study. Clinical, pathologic and immunohistochemical data are summarized in [Table/Fig-1]. Luminal A breast carcinoma cases comprised 35.8% of the cases, while the breakdown percentage of cases that were Luminal B, HER-2/neu positive and TNBC were 21%, 19.8% and 23.5% respectively.
Clinical, pathologic and immunohistochemical results of the studied 81 breast carcinoma cases.
| Variables | Number (%) |
|---|
| Age |
| ≤50 | 45 (55.6) |
| >50 | 36 (44.4) |
| Menopausal status |
| Perimenopausal | 33 (40.7) |
| Post-menopausal | 48(59.3) |
| Tumour size (cm) |
| ≤2 | 24 (29.6) |
| >2 | 57 (70.4) |
| Histologic type |
| IC,NST | 75 (92.6) |
| ILC | 6 (7.4) |
| Histologic grade |
| Grade I | 6 (7.4) |
| Grade II | 39 (48.1) |
| Grade III | 36 (44.4) |
| In-situ component |
| Negative | 36 (44.4) |
| Positive | 45 (55.6) |
| DCIS type |
| Comedo | 15 (18.5) |
| Non-comedo | 66 (81.5) |
| LVI |
| Negative | 60 (74.1) |
| Positive | 21(25.9) |
| Nodal metastasis |
| Negative | 18 (22.2) |
| Positive | 63 (77.8) |
| Stage |
| Early | 31 (38.3) |
| Late | 50 (61.7) |
| NPI |
| Good-moderate | 36 (44.4) |
| Poor | 45(55.6) |
| ER |
| Negative | 35(43.2) |
| Positive | 46(56.8) |
| PR |
| Negative | 40(49.4) |
| Positive | 41(50.6) |
| Her-2/neu |
| Negative | 48(59.3) |
| Positive | 33(40.7) |
| Ki-67 LI |
| Negative | 30(37) |
| Positive | 51(63) |
| Molecular subtype |
| Luminal A | 29(35.8) |
| Luminal B | 17(21) |
| Her-2/neu positive | 16(19.8) |
| TNBC | 19(23.5) |
IC-NST: invasive carcinoma-no special type; ILC: invasive lobular carcinoma; DCIS: duct carcinoma insitu; LVI: lymphovascular invasion; NPI: Nottingham prognostic index; ER: estrogen receptor; PR: progesterone receptor, Ki-67 LI: Ki-67 labeling index; TNBC: triple negative breast carcinoma
AR Immunostaining
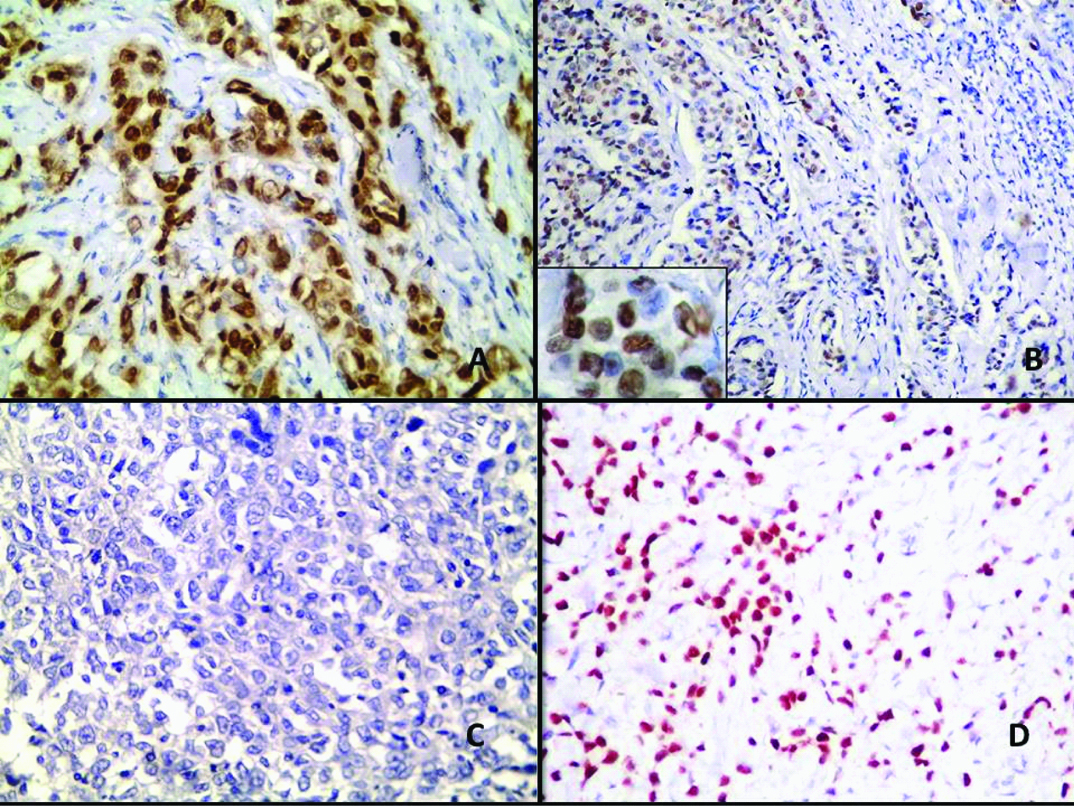
In the current study, (30/81) 37.04% of BC cases showed AR immunoreactivity. Most AR positive cases (60%) were seen in older women (p=0.03) and 83.3% were seen in post-menopausal women (p=0.001). 76.7% of the AR positive cases were of grade I -II (p=0.003). AR immunoreactivity was also related to the presence of in-situ component (p= 0.014), although type of in-situ component doesn’t show statistical difference. An early stage at presentation and good-moderate NPI (p=0.03 and 0.009 respectively) showed significant relationship with AR immunoreactivity. Positive ER, negative HER-2/neu, low Ki-67 proliferation index and Luminal A subtype showed significant association with AR expression as well [Table/Fig-2]. Immunostaining of BC is shown in [Table/Fig-3,4].
Relationship between AR expression in BC cases and clinicopathologic variables.
| Variables | AR expression | Test of significance | p-value |
|---|
| NegativeNumber (%)51 (62.96) | PositiveNumber (%)30 (37.04) |
|---|
| Age |
| ≤ 50 | 33(64.7%) | 12(40%) | X2 = 4.69 | 0.03 |
| >50 | 18(35.3%) | 18(60%) |
| Menopausal status |
| Perimenopausal | 28(54.9%) | 5(16.7%) | X2 = 11.4 | 0.001 |
| Post-menopausal | 23(45.1%) | 25(83.3%) |
| Tumour size (cm) |
| ≤ 2 | 14(27.5%) | 10(33.3%) | X2 = 0.31 | 0.37 |
| >2 | 37(72.5%) | 20(66.7%) |
| Histologic type |
| IC,NST | 49(96.1%) | 26(86.7%) | X2 = 2.44 | 0.118 |
| ILC | 2(3.9%) | 4(13.3%) |
| Histologic grade |
| Grade I-II | 22(43 .1%) | 23(76.7%) | X2 = 8.6 | 0.003 |
| Grade III | 29(56.9%) | 7(23.3%) |
| In-situ component |
| Negative | 28(54.9%) | 8(26.7%) | X2 = 6.09 | 0.014 |
| Positive | 23(45.1%) | 22(73.3%) |
| DCIS type |
| Comedo | 6(26.1%) | 9(40.9%) | X2 = 1.112 | 0.23 |
| Non comedo | 17(73.9%) | 13(59.1%) |
| LVI |
| Negative | 38(74.5%) | 22(73.3%) | X2 = 0.014 | 0.55 |
| Positive | 13(25.5%) | 8(26.7%) |
| Nodal metastasis |
| Negative | 8(15.7%) | 10(33.3%) | X2 = 3.4 | 0.7 |
| Positive | 43(84.3%) | 20(66.7%) |
| Stage |
| Early | 15(29.4%) | 22(73.3%) | X2 = 4.57 | 0.03 |
| Late | 36(70.6%) | 8(26.7%) |
| NPI |
| Good-moderate | 17(33.3%) | 19(63.3%) | X2 = 6.89 | 0.009 |
| Poor | 34(66.7%) | 11(36.7%) |
| ER |
| Negative | 26(51%) | 9(30%) | X2 = 3.38 | 0.05 |
| Positive | 25(49) | 21(70%) |
| PR |
| Negative | 29(56.9%) | 11(36.7%) | X2 = 3.08 | 0.06 |
| Positive | 22(43.1%) | 19(63.3%) |
| Her-2/neu |
| Negative | 23(45.1%) | 25(83.3%) | X2 = 11.43 | 0.001 |
| Positive | 28(54.9%) | 5(16.7%) |
| Ki-67 LI |
| Low | 10(19.6%) | 20(66.7%) | X2 = 17.9 | 0.001 |
| High | 41(80.4%) | 10(33.3%) |
| Molecular subtype |
| Luminal A | 8(15.7%) | 21(70%) | X2 = 25.7 | 0.001 |
| Luminal B | 16(31.4%) | 1(3.33%) |
| Her-2/neu positive | 12(23.5%) | 4(13.3%) |
| TNBC | 15(29.4%) | 4(13.3%) |
| Luminal vs non luminal |
| Luminal | 24(47.1%) | 22(73.3%) | X2 = 5.31 | 0.02 |
| Non luminal | 27(52.9%) | 8(26.6%) |
IC, NST, Invasive Carcinoma, no special type; ILC, Invasive Lobular Carcinoma; DCIS, Duct Carcinoma Insitu; LVI, Lymphovascular Invasion; NPI, Nottingham Prognostic Index; ER, Estrogen Receptor; PR, Progesterone Receptor, Ki-67 LI, Ki-67 Labeling Index; TNBC, triple Negative Breast Carcinoma. X2; Chi-square test
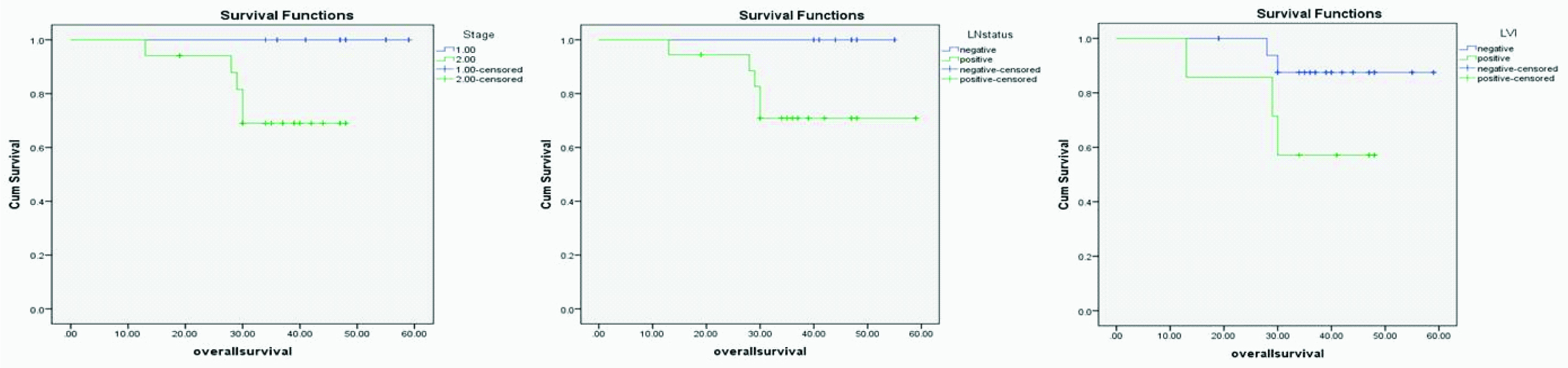
Kaplan-Meier Overall Survival (OS) for stage, lymph node status and LVI (p=0.005, 0.013, and 0.003).
Four different cases of BC; (a&b) are cases of IC-NST showing positive nuclear staining for AR (IHC of AR X400 and X100 respectively, inset X 400). A case of IC-NST showing negative AR expression; (c) (IHC of AR X400). A case of ILC with positive nuclear AR staining; (d) (IHC of AR X400).
Survival Analysis
The mean OS for BC patients was 36.3 (range of 13-59 months). The univariate analysis of the OS for patients involved in the current study showed that each of earlier stage (Log rank of 7.77 and p=0.005), negative lymph nodes (Log rank =6.22 and p=0.013), and negativeLVI (Log rank =8.75 and p=0.003) are associated with longer OS in BC patients [Table/Fig-3].
Discussion
BC is a heterogeneous hormone-dependent tumour with distinctive molecular subtypes. AR is thought to play a role in BC development and to be correlated with BC clinicopathologic variables with variable role in different molecular types [5].
In the current study, we sought to address the relationship between AR expression and different clinico-pathologic variables, molecular biomarker expression and molecular groups in BC of Egyptian patients. The correlation between AR and the patient’s OS was studied as well.
Studying the relationship between AR expression and clinical variables in BC patients; it was found that AR was positive in 37.04% of cases, which is lower than the levels noted in other populations [5] and [15], propably explaining the poor prognostic nature of the disease in Egyptian patients. AR immunoreactivity was related significantly to patient’s age and post-menopausal state (p=0.03 and 0.001 respectively), which was similarly observed in previous studies [16]. A recent study proposed a novel approach of BC prevention through reducing androgen production in menopausal females [17].
Other immunohistologic variables were evaluated as well as in relation to AR expression in this group of patients and although no significant correlation was found between AR immunoreactivity and histologic type of tumour, a significant relationship was found between its expression and grades I-II, the breakdown of AR positive cases among histologic grade was as follows; 76.6% in I-II, and 32.3% in the poorly differentiated grade. Similar results were observed by Tokunaga et al., who observed a correlation between AR expression and lower nuclear grade tumours in ER positive BC. He found that AR immunoreactivity was related to other known good prognostic variables in breast carcinoma. It is related to good-moderate NPI (p=0.009) and early BC stage (p=0.03) [18].
In this study, the correlation between AR expression and the molecular subtype of BC revealed that, AR was expressed in significantly higher proportions of luminal breast carcinoma cases (73.3%). AR immunoreactive cases were split among all subtypes of breast carcinoma as follows 70%, 3.33%, 13.3% and 13.3% in Luminal A, Luminal B, HER-2/neu, and TNBC respectively. Unsurprisingly, AR immunoreactivity was also related significantly to ER positivity (p=0.05), negative HER2/neu (0.001), and low Ki-67 index(0.001). Results obtained in this study were not different from other studies. Tokunaga found AR immnoreactivity to be associated with ER, PR, low Ki-67, and Her-2/neu negativity as well as lower nuclear grades [18]. Similarly, it was also found that AR is expressed in luminal more than non-luminal BC cases, and it was related to low Ki-67 proliferative index [19].
Based on the above results, AR immunoreactivity is significantly related mostly to luminal ER positive cases, an observation that can explain the relationship between AR positivity and the better clinicopathologic features in terms of prognosis.
The role of Androgen signaling in neoplastic cells remains controversial. It is involved in normal breast development [20]. Nevertheless, androgens influence the risk of BC through different contradictory mechanisms: either by AR binding which stimulates malignant cell proliferation, or through binding to ER with subsequent competitive inhibition of 17 b-estradiol stimulatory effect on neoplastic cells, or by conversion to estradiol [21].
Despite the strong evidence of the favourable prognostic role of AR in BC, butin univariate analysis. AR immunreactivity, was not correlated with OS in this study. Earlier stage of presentation, less number of involved nodes and negative LVI are the only factors that were associated with longer OS in BC cases.
The prognostic role of AR in BC is still controversial with variable results. Castello et al., have found AR to be a good prognostic factor in ER + BC cases [22]. On the other side, Hu et al., has found AR to be associated with poor survival in post-menopausal women [23]. Classifying BC cases into ER + and ER- cases or to pre-menopausal and post-menopausal categories could reveal more data about the effect of AR on BC survival in Egyptian patients.
In our study 19% of cases were TNBC and AR was expressed in 13.3% of them. TNBC percentages of BC was variable among studies from 7% to 60% [24,25]. Percentage of AR positive TNBC also caries from 6.6% to 75% [26,27]. TNBC could be split into AR positive and AR negative tumours, most AR positive TNBC cases are found to be apocrine carcinomas [28,29].
AR-antagonists could be a novel therapeutic targets, particularly for those patients with TNBC due to the futility of conventional anti-hormonal and anti-HER2 therapy. Naderi et al., found invivo and invitro synergies between AR and MEK inhibitors in apocrine breast carcinoma, their combination could overcome trastuzymab resistance [30]. They suggested that a combination strategy of therapy may provide an attractive therapeutic options for the ER-/AR+ subtypes of breast carcinoma.
Limitation
Studying the role of AR in each of the molecular subtypes on a larger population is recommended to further elucidate the role of AR in different molecular subtypes of BC.
Conclusion
AR is found to be related to favourable prognostic factors in BC but not correlated with OS in studied cases. It is expressed in all types of BC particularly in Luminal A group and in significant proportion in TNBC, which offers an opportunity for AR being used as a targeted therapy for these patients.