Revitalization of an Immature Permanent Mandibular Molar with a Necrotic Pulp Using Platelet-Rich Fibrin: A Case Report
Dayalan Subash1, Krishnamma Shoba2, Shibu Aman3, Srinivasan Kumar Indu Bharkavi4
1 Senior Resident, Department of Conservative Dentistry and Endodontics, Government Dental College, Kottayam, Kerala, India.
2 Professor and Head, Department of Conservative Dentistry and Endodontics, Government Dental College, Kottyam, Kerala, India.
3 Assisant Professor, Department of Conservative Dentistry and Endodontics, Government Dental College, Kottyam, Kerala, India.
4 Senior Lecturer, Department of Oral Pathology and Microbiology, Sathyabama University Dental College and Hospital, Chennai, Tamil Nadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Dayalan Subash, Senior Resident, Department of Conservative Dentistry and Endodontics, Government Dental College, Kottayam, Gandhi Nagar, P.O., Kottayam-686008, Kerala, India.
E-mail: dr.subash06@yahoo.com
Any insult to the pulp during its development causes cessation of dentin formation and root growth. Pulpal status and degree of root development are the decisive factors in the treatment approach. Various treatment options have been tried like surgery with root-end sealing, calcium hydroxide–apexification, placement of apical plug and regenerative endodontic procedures to induce apexogenesis. An ideal scenario for a necrosed tooth with immature root would be continued root development coupled with regeneration of pulp tissue. We report a case, where revitalization was done using Platelet-Rich Fibrin (PRF) as a scaffold in immature mandibular molar tooth.
Biodentine, Biomimetic, Open apex, Regenerative endodontics, Revascularisation
Case Report
A 13-year-old male reported with a chief compliant of pain in the left posterior tooth region reported to the Department of Conservative Dentistry and Endodontics. The pain was intermittent, dull in nature for the past one month. History revealed that the pain started three months previously, which was continuous in nature for which he underwent treatment elsewhere, but did not complete it. There was no relevant medical history. Clinical examination revealed a temporary restoration in the second left mandibular molar (tooth #37). The tooth was sensitive to both percussion and palpation tests. It did not respond to cold (Endo Ice, Coltene Whaledent, Ohio, USA) and Electric Pulp Tester (EPT). The contralateral tooth was negative to percussion and palpation tests and responded positively to cold and EPT. Periodontal probing depths were within normal limits. Radiographic examination revealed incomplete endodontic treatment with immature root and wide open apex in tooth #37 [Table/Fig-1a], periapical radiolucencies were also noted around the apices. On the basis of clinical and radiographic findings, a diagnosis of necrotic pulp and periapical diagnosis of symptomatic apical periodontitis were confirmed. So, a regenerative endodontic procedure was planned with the aid of Platelet-Rich Fibrin (PRF).
a) Pre-operative intra-oral periapical radiograph showing wide root canal with open apex in mandibular molar tooth; b) PRF clot retrieved with the help of tweezers and placed on modified endo box for membrane preparation; c) Autologous fibrin membrane prepared; d) Fibrin membrane fragmented.
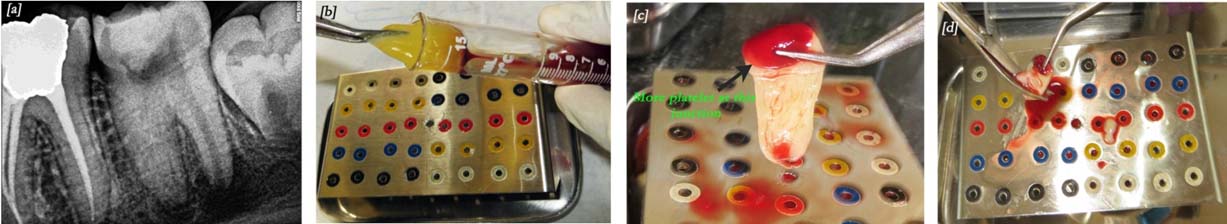
An informed consent was obtained from parents. Local anesthesia (2% lidocaine with 1:100,000 epinephrine) was administered. Rubber dam was applied and temporary restoration removed on tooth #37. Three canals were negotiated (mesio-buccal, mesio-lingual and distal). Working length was determined radiographically. Cleaning and shaping was performed in the mesial canals up to ProTaper F3 file (Dentsply, Pennsilvania, USA), to facilitate PRF placement. The canal was irrigated with approximately 10ml of 5.25% sodium hypochlorite, then neutralized with saline and dried with paper points. Triple antibiotic paste was prepared with mixing equal proportions of ciprofloxacin (Ranbaxy Lab, India), metronidazole (Unique Pharmaceuticals, Mumbai, India) and minocycline (Aurobindo, Andhra Pradesh, India) with propylene glycol into a thick paste. This antibiotic mixture was placed into the canals using a lentulo spiral up to the canal orifice. A cotton pellet was placed inside the pulp chamber and access cavity sealed with Cavit (ESPE, Cergy Pontoise, France).
The patient reported after 21 days without any symptoms. A 10ml sample of whole blood was drawn from the patient’s right arm. The blood sample was transferred into a test tube without anti-coagulant and centrifuged immediately (REMI Model R-8c, India) at 3000rpm for 10 minutes to obtain the PRF. After centrifugation, PRF was removed from the test tube with the help of sterile tweezers [Table/Fig-1b]. Jelly-like PRF was converted into membrane in a modified endodontic file box [Table/Fig-1c]. This fibrin membrane was fragmented into small pieces with the help of surgical scissors [Table/Fig-1d]. So, that it can be easily placed inside the canals. In the mean time, local anaesthesia was given. After rubber dam isolation, the access cavity was reopened and saline irrigation done to remove the antibiotic mixture [Table/Fig-2a]. Each canal was dried with paper points and made ready for the placement of PRF.
Clinical procedures; a) Canals after saline irrigation, dried and kept ready for PRF placement; b) Fibrin membrane placed until the roof of the pulp chamber; c) Cavity restored with biodentine.
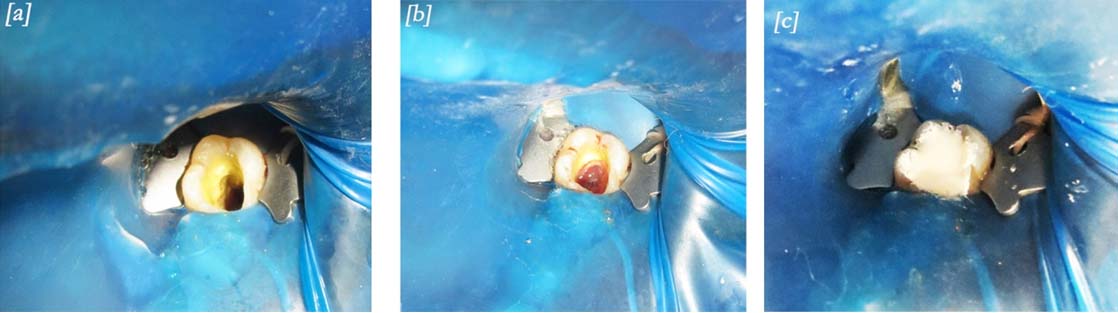
The fragmented membrane was placed in the pulp chamber and pushed apically with the help of endodontic pluggers. This process was continued until the roof of the pulp chamber was filled with PRF [Table/Fig-2b]. Biodentine (Septodont, France) was placed directly over the fibrin clot and the reminder of the cavity was also restored fully with it [Table/Fig-2c]. During the 1st month follow-up the Biodentine was partially removed and a composite restoration placed over it.
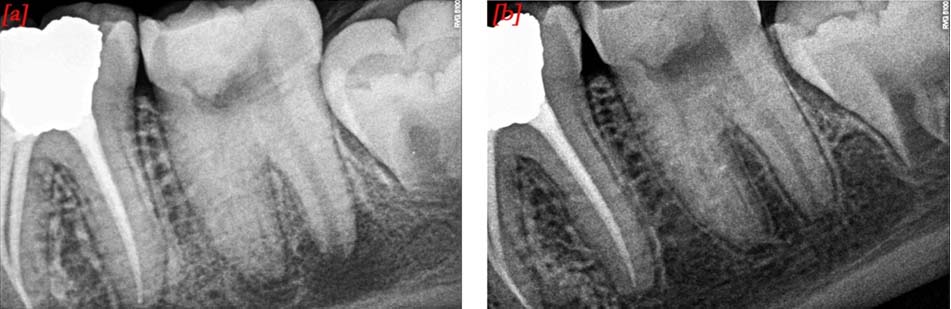
The patient was reviewed at 3, 6, 9 and 12 months. The tooth# 37 was asymptomatic and was not sensitive to percussion. On the 9th month review, pulp sensibility tests with cold and EPT, elicited a positive response which was comparable to the contralateral molars. Radiographic examination revealed resolution of periapical lesion, thickening of the dentinal walls, root lengthening with apical closure showing a continuous lamina dura [Table/Fig-3a,b].
Radiographs; a) At 6th month follow-up, tooth showing decreased periapical radiolucency and increased root length; b) At 12th month follow-up, tooth showing resolution of periapical lesion, thickening of the dentinal walls, root lengthening with apical closure showing continuous lamina dura.
Discussion
Regenerative endodontics involves biological procedures directed towards replacement of damaged structures of the pulp-dentin complex [1]. Several case reports including the present study demonstrated that, teeth with necrotic pulp and immature apices are capable of regenerating tissues of pulp-dentin complex, continue root maturation with closure of apex and can regain tooth vitality with proper disinfection and a suitable scaffold.
Hargreaves KM et al., proposed “Regeneration of pulp-dentin complex” methods resulted in restoration of some of the functional properties of involved teeth [2]. Clinically successful regeneration can be attained by complete disinfection, an ideal scaffold and bio-interactive material with tight seal. In our case, disinfection was obtained by the use of 5.25% sodium hypochlorite as an irrigant and triple antibiotic paste as an intra-canal medicament as suggested by various researchers [3–5]. Shaping of mesial canals was done merely to create space for the placement of PRF to the near apical region. In our case, care was taken to ensure that triple antiobiotic paste placed till the canal orifice without extending in to the pulp chamber, to avoid the chance of staining from minocycline [6].
After disinfection, the next component needed was a physical scaffold which supports cell growth and differentiation, supply signalling molecules and growth factors for the maturation and differentiation of stem cells [2,3]. Previous case reports [5,7], have shown that PRF can be used as a scaffold. All case reports done in the past were on anterior teeth with good success. Instead in our study PRF was placed in a second molar, where the accessibility is very minimal for placement of membrane to the apex. The PRF membrane was fragmented for easy placement to the apical area. No bleeding was noted during the placement. The pulp chamber was also filled with PRF, to facilitate the regeneration till that area, so that reliable results can be achieved with pulp sensibility tests.
PRF is a second generation platelet concentrate with a simplified preparation technique and genuinely autologous in nature. It is an accumulation of platelets and cytokines in a fibrin clot. The platelets concentrate mainly at the junction between the red corpuscles (red thrombus) and the PRF clot in the test tube. This platelet component of PRF is considered vital for regeneration [Table/Fig-1d]. PRF has several advantages over other platelet concentrates. It enmeshes glycosaminoglycans, which in turn attracts platelet cytokines and also has greater capacity for cell migration.
The PRF releases high quantities of growth factors like transforming growth factor beta-1, platelet derived growth factor AB, vascular endothelial growth factor and glycoprotein, which stimulates cell migration and proliferation [8,9]. PRF stimulates cell proliferation and differentiation of dental pulp cells by up-regulating osteoprotegerin and alkaline phosphatase expression [10]. PRF offers a three-dimensional fibrin network with flexible architecture facilitating easy placement thereby making it an excellent substrate for cell growth.
Unlike previous studies, Biodentine was directly placed over the PRF clot to obtain a tight seal and avoid bacterial contamination. Biodentine possess enhanced properties such as quick setting time (in comparison with MTA), Good marginal adapatation [11] and high strength not usually associated with other tricalcium cements [12]. Chang SW et al., suggested that this could be useful for regenerative endodontic procedures, since it stimulates odontoblastic differentiation and nodule formation during minera-lisation [13]. Because of its high strength and sealing ability, cavity was fully filled with biodentine. Subsequently composite was placed over Biodentine at the first month follow-up visit.
At the end of 1 year follow-up, the tooth was responding positively to pulp sensibility tests and the radiographic examination revealed resolution of periapical lesion, thickening of the dentinal walls, root lengthening with apical closure showing continuous lamina dura [Table/Fig-3a-c]. The reason for these findings might be mesenchymal stem cells of apical papilla differentiating into various cells for matrix secretion along with activation of osteoblasts, fibroblasts, endothelial cells and epithelial cells. This effect can be due to cytokines and growth factors released from PRF [1,14]. Evidence have shown that, pulp-like tissue can be regenerated with the use of platelet concentrate [15]. The successful outcome of this case can be attributed to the formation of pulp-like tissue and cell differentiation. The exact nature of the tissue generated can be studied only by histological examination.
Conclusion
The trio of disinfection with triple antibiotic paste, PRF as a scaffold and Biodentine as a bio-interactive material seems to be a viable combination for pursuing regenerative treatment procedures especially in molars. Our study opens up new frontiers for revitalisation procedures in posterior teeth where access was considered difficult. More clinical trials are warranted to achieve success on a consistent basis on posterior teeth.
[1]. Murray PE, Garcia-Godoy F, Hargreaves KM, Regenerative endodontics: A review of current status and a call for actionJ Endod 2007 33(4):377-90. [Google Scholar]
[2]. Hargreaves KM, Giesler T, Henry M, Wang Y, Regeneration potential of the young permanent tooth: What does the future hold?J Endod 2008 34:S51-56. [Google Scholar]
[3]. Ding RY, Cheung GS, Chen J, Yin XZ, Wang QQ, Zhang CF, Pulp revascularization of immature teeth with apical periodontitis: A clinical studyJ Endod 2009 35(5):745-49. [Google Scholar]
[4]. Torabinejad M, Turman M, Revitalization of tooth with necrotic pulp and open apex by using platelet-rich plasma: A case reportJ Endod 2011 37:265-68. [Google Scholar]
[5]. Shivashankar VY, Johns DA, Vidyanath S, Kumar MR, Platelet rich fibrin in the revitalization of tooth with necrotic pulp and open apexJ Conserv Dent 2012 15(4):395-98. [Google Scholar]
[6]. Kim JH, Kim Y, Shin SJ, Park JW, Jung IY, Tooth discoloration of immature permanent incisor associated with triple antibiotic therapy: A case reportJ Endod 2010 36(6):1086-91. [Google Scholar]
[7]. Keswani D, Pandey RK, Revascularization of an immature tooth with a necrotic pulp using platelet-rich fibrin: A case reportInt Endod J 2013 46(11):1096-104. [Google Scholar]
[8]. Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJJ, Mouhyi J, Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part II: Platelet-related biologic featuresOral Surg Oral Med Oral Pathol Oral Radiol Endod 2006 101(3):e45-50. [Google Scholar]
[9]. Dohan Ehrenfest DM, de Peppo GM, Doglioli P, Sammartino G, Slow release of growth factors and thrombospondin-1 in Choukroun’s platelet-rich fibrin (PRF): A gold standard to achieve for all surgical platelet concentrates technologiesGrowth Factors 2009 27(1):63-69. [Google Scholar]
[10]. Huang FM, Yang SF, Zhao JH, Chang YC, Platelet-rich fibrin increases proliferation and differentiation of human dental pulp cellsJ Endod 2010 36:1628-32. [Google Scholar]
[11]. Ravichandra PV, Harikumar Vemisetty, Deepthi K, Jayaprada Reddy S, Ramkiran D, Jaya Nagendra Krishna M, Comparative evaluation of marginal adaptation of biodentine(tm) and other commonly used root end filling materials-An in-vitro studyJ Clin Diagn Res 2014 8(3):243-45. [Google Scholar]
[12]. Grech L, Mallia B, Camilleri J, Characterization of set intermediate restorative material, biodentine, bioaggregate and a prototype calcium silicate cement for use as root-end filling materialsInt Endod J 2013 46(7):632-41. [Google Scholar]
[13]. Chang SW, Lee SY, Ann HJ, Kum KY, Kim EC, Effects of calcium silicate endodontic cements on biocompatibility and mineralization-inducing potentials in human dental pulp cellsJ Endod 2014 40(8):1194-200. [Google Scholar]
[14]. Lin LM, Ricucci D, Huang GT, Regeneration of the dentine – Pulp complex with revitalization/revascularization therapy: Challenges and hopesInt Endod J 2014 47:713-24. [Google Scholar]
[15]. Torabinejad M, Faras H, A clinical and histological report of a tooth with an open apex treated with regenerative endodontics using platelet-rich plasmaJ Endod 2012 38:864-68. [Google Scholar]