Stroke causes a variety of impairments that compromise quality of life. Involvement of upper limb as a component of hemiparesis is one such commonly encountered impairment and is particularly problematic in managing Activities of Daily Living (ADL) [1,2]. Various rehabilitation methods have been applied to improve hemiparesis with varying success [3,4].
As a result of spontaneous recovery and rehabilitation, upper limb weakness gradually improves in many patients but the actual use of arm for function is often less than the potential use [5]. This was explained by learned non-use theory, according to which repeated failure in attempting the use of affected arm during the acute phase of stroke leads to negative enforcement of paretic arm use [6,7]. This can be reverted back by forced use of arm [8].
Constraint Induced Movement Therapy (CIMT) is a fairly new technique used in rehabilitation medicine to treat individuals with decreased upper extremity function [6]. CIMT emphasizes massed practice with the affected upper limb. This is accomplished by restraining the subjects’ less affected upper limb and training of affected limb by shaping movements [9]. These repetitive movements of the affected limb in CIMT induce cortical reorganization. Following CIMT, gains in motor function with accompanying neuroplastic changes in function and structure have been demonstrated in stroke patients [10,11]. The original CIMT devotes six or more hours for therapy and constraining of the intact arm for 90% of waking hours per day and over a period of two weeks. Researchers have observed that such a schedule of CIMT is exhaustive and possibly resulting in non-compliance [12]. Modified, shorter versions of CIMT (mCIMT) have been designed and tried to overcome such limitations [12–16]. In literature, large variety of mCIMT paradigm has been reported. Duration of intervention varies from 2 to 10 weeks and the treatment time also varies from as short as 30 minutes to three hours per day in various studies reviewed by us [17–22]. Nevertheless both CIMT and mCIMT have shown promising success [9,23,24].
In this study we have made an attempt to investigate the efficacy of mCIMT with a therapy time of three hours per day for three days per week and a constraint time of five hours per day for five days per week over duration of four weeks. The objectives of the study were to compare the efficacy of combination of mCIMT and conventional rehabilitation program (study group) and conventional rehabilitation program alone (control group) in improving the hand function of stroke patients in terms of motor recovery and functional outcome.
Materials and Methods
This prospective single blind, parallel randomized controlled trial in the management of hemiparetic upper limb following stroke was carried out during the period from October 2010 to April 2012. All the patients of stroke with hemiparesis presenting to outpatient Department of Physical Medicine and Rehabilitation at VMMC and Safdarjung Hospital were examined and screened for inclusion in the study. Post stroke hemiparetic patients of two months to two years duration with spasticity ≤ Grade -3 on modified Ashworth scale and those patients capable of extension of at least 10° each at Metacarpophalangeal (MCP), Proximal Interphalangeal (PIP) and Distal Interphalangeal (DIP) joints and 20° at wrist joint were recruited for the study after obtaining written informed consent [6,14,25]. Patients with history of previous stroke, angina, uncontrolled hypertension, on medication that could impair neuromuscular performance, with wrist or finger pathologies, significant visual or hearing impairment, balance problems which may compromise safety during sound upper limb constraint, and those unwilling to participate in the study were excluded from the study.
On the basis of previous study, mCIMT produced significant and large effects on Motor Activity Log (MAL) and Fugl-Meyer Assessment (FMA) scores [14,17,19]. To detect large scale effect size ES (.75), the minimum required sample size with 80% power of study and two sided alpha of 5% was 28 patients per group. So sample size taken was 60(30 per group). Formula used was n ≥ 2{(Zα + Zβ)2/(ES)2} where Zα (=1.96) is value of Z at two sided alpha error of 5% and Zβ(=0.86) is value of Z at power of 80% and ES is effect size.
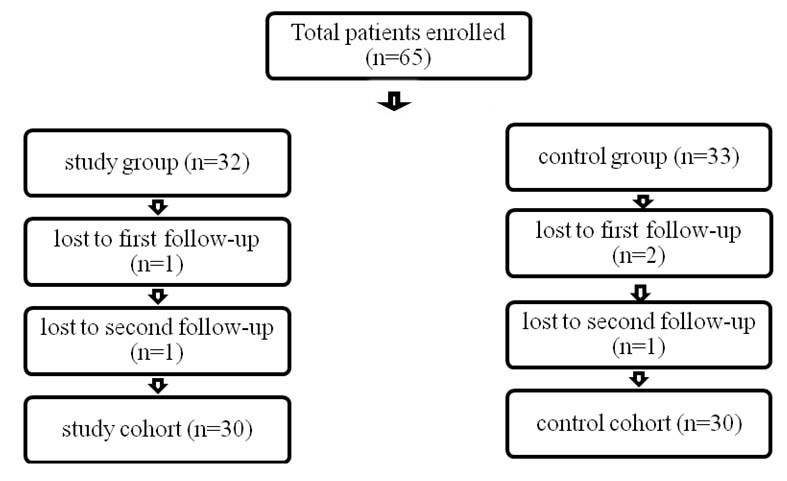
Sixty five patients fulfilled the criteria and were enrolled in the study but total 60 patients completed the study [Table/Fig-1]. Complete work up of each patient was done including history, physical examination and relevant investigations. Patients were randomly assigned, by lots drawn, to either study group or control group. The control group received 3 hours daily of conventional rehabilitation programme which included ADL training, stretching, range of motion and strengthening exercises, endurance training, gait training, orthosis, and education, as appropriate. The study group participated in a modified CIMT programme in addition to the conventional rehabilitation programme. Patients in this group were asked to do activities of either mCIMT or conventional rehabilitation programme on a particular day. Therapy sessions during mCIMT concentrated on affected limb use, in functional tasks like reaching forward to hold a glass and drinking from it, picking up a comb and combing hair, turning on and off a light switch, buttoning and unbuttoning of clothes, writing with a pen. This was done for three hours in a day alternatively for three days a week. A constraint session of the unaffected limb was also used for five hours per day for five days a week. For the constraint session the patient’s unaffected hand and wrist was covered with a mitt during times of frequent arm use and during activities of mCIMT. The total duration of intervention was four weeks.
Flow diagram of the patients distributed in the two groups.
All the patients were assessed at the beginning of the study (baseline), at four weeks (first follow-up i.e., at the time of completion of therapy) and at three months after completion of therapy (second follow-up) using FMA for motor recovery of upper extremity and MAL scale.
FMA for motor recovery of upper extremity is a motor performance test consisting of 33 tasks performed by the affected upper extremity, evaluating the ability to make movements outside of a synergistic pattern [26]. Performance on each task is given a score of 0, 1, or 2. Higher score represent better performance (possible range 0 to 66). The MAL evaluates how the affected hand is used to perform activities of daily living [27]. For each activity, the patient rates how much the affected hand is used i.e., Amount of Use Score (AOUS) and how well the activity is performed i.e., Quality of Use Score (QOUS). Ratings are on a scale from 0 to 4, with higher scores representing better function.
Categorical variables were presented in number and percentage (%) and continuous variables were presented as mean ± SD and median. Qualitative variables were compared using chi-square test. Quantitative variables were compared using Independent t-test for parametric analyses and Mann-Whitney U Test for non-parametric analyses. ANCOVA was used to compare FMAS, QOUS, AOUS at one month and three months between two groups after adjusting for the baseline values. Repeated measure ANOVA was used for comparison between 0 week, one month and three months and Boneferroni’s correction was used for pairwise comparison within the group. A p-value of <0.05 was considered statistically significant. The data was entered in MS EXCEL spreadsheet and analysis was done using Statistical Package for Social Sciences (SPSS) version 21.0. The study was cleared ethically from the Institutional Ethical Committee board.
Results
A total number of 65 subjects satisfying the inclusion criteria were enrolled in the study. Out of the total subjects enrolled, 60 patients (30 in each group) completed the three month follow-up period; three subjects did not come for first follow-up while two did not attend the second follow-up [Table/Fig-1].
Baseline characteristics of both groups were comparable. Qualitative variables gender, literacy rate, income status, side involved and cause of stroke were compared using chi-square test [Table/Fig-2]. Quantitative variables age, duration of stroke, FMAS, QOUS, AOUS at 0 weeks, minimum active extension at PIP and DIP were parametric so Independent t-test was used while minimum active extension at wrist and MCP were non parametric, so Mann Whitney U was used for comparison [Table/Fig-3].
Baseline characteristics with qualitative variables.
| Characteristics | Study group (n=30) | Control group (n=30) | p- value | Chi-Square | Df |
|---|
| Sex (M:F) | 20:10 | 24:6 | 0.243 | 1.364 | 1 |
| Literacy (illiterate:primary: secondary: highersecondary: graduate: postgraduate) | 8:8:7: 4:3:0 | 6:6:6: 5:6:1 | 0.737 | 2.759 | 5 |
| Income (≤5000:≤10000:≤15000:>15000) | 17:9: 2:2 | 13:9:4:4 | 0.6005 | 1.867 | 3 |
| Hemiplegic side (left: right) | 23:07 | 22:08 | 0.766 | 0.089 | 1 |
| Cause of Stroke(infarction: hemorrhage) | 20:10 | 18:12 | 0.592 | 0.287 | 1 |
Baseline characteristics with quantitative variables.
| Study group | Control group | p-value |
|---|
| Age in years |
| Mean ± SD | 47.03 ± 13.76 | 46.3 ± 13.6 | 0.8363 |
| Median | 46.5 | 47 |
| Min-Max | 22-78 | 21-73 |
| Duration in months |
| Mean ± SD | 10.07 ± 6.21 | 10.18 ± 6.17 | 0.9459 |
| Median | 8.67 | 9.42 |
| Min-Max | 2.5-23 | 2.23-23 |
| Minimum active extension at wrist in degrees |
| Mean ± SD | 52.17 ± 17.15 | 52.83 ± 15.46 | 0.9167 |
| Median | 57.5 | 52.5 |
| Min-Max | 20-80 | 20-75 |
| Minimum active extension at MCP in degrees |
| Mean ± SD | 48.17 ± 20.4 | 50.33 ± 15.48 | 0.8467 |
| Median | 50 | 50 |
| Min-Max | 10-80 | 15-70 |
| Minimum active extension at PIP in degrees |
| Mean ± SD | 45.07 ± 17.96 | 44.33 ± 15.35 | 0.8656 |
| Median | 50 | 47.5 |
| Min-Max | 10-80 | 15-70 |
| Minimum active extension at DIP in degrees |
| Mean ± SD | 44.17 ± 18.2 | 44.5 ± 14.99 | 0.9385 |
| Median | 47.5 | 45 |
| Min-Max | 10-80 | 15-70 |
| FMAS0 |
| Mean ± SD | 34.67 ± 3.55 | 34.7 ± 3.19 | 0.9696 |
| Median | 35.5 | 35 |
| Min-Max | 28-40 | 28-40 |
| AOUS0 |
| Mean ± SD | 11.5 ± 2.15 | 11.4 ± 2.09 | 0.8557 |
| Median | 12 | 11.5 |
| Min-Max | 8-15 | 8-15 |
| QOUS0 |
| Mean ± SD | 10.43 ± 2.06 | 10.2 ± 1.95 | 0.6546 |
| Median | 10.5 | 10 |
| Min-Max | 7-15 | 7-14 |
Independent t test applied except minimum active extension at wrist and MCP criteria for which Mann Whitney U test applied.
Both the groups showed improvement in the study. All the three scores improved significantly at one month (FMAS1, AOUS1, QOUS1) and three months (FMAS3, AOUS3, QOUS3) as shown in [Table/Fig-4].
Improvements in FAMS, AOUS and QOUS at 1 month and 3 months in two groups., Mann Whitney U test applied.
| Study group | Control group | |
|---|
| Improvement in FMAS1 |
| Mean ± SD | 13.4 ± 2.97 | 10.7 ± 1.24 | <.0001 |
| Median | 14 | 11 |
| Min-Max | 1-17 | 7-13 |
| Improvement in AOUS1 |
| Mean ± SD | 6.57 ± 1.3 | 5.47 ± 1.04 | 0.0001 |
| Median | 6.5 | 5.5 |
| Min-Max | 2-9 | 4-9 |
| Improvement in QOUS1 |
| Mean ± SD | 6.37 ± 1.35 | 5.3 ± 1.06 | 0.0002 |
| Median | 7 | 5 |
| Min-Max | 2-8 | 4-9 |
| Improvement in FMAS3 |
| Mean ± SD | 15.9 ± 2.78 | 12.2 ± 1.38 | <.0001 |
| Median | 17 | 12 |
| Min-Max | 4-20 | 9-16 |
| Improvement in AOUS3 |
| Mean ± SD | 8.2 ± 1.71 | 6.63 ± 1.43 | <.0001 |
| Median | 8 | 7 |
| Min-Max | 3-13 | 2-11 |
| Improvement in QOUS3 |
| Mean ± SD | 7.77 ± 1.5 | 6.53 ± 1.14 | 0.0001 |
| Median | 8 | 6.5 |
| Min-Max | 3-11 | 4-10 |
In both the study group and control group, a repeated measures ANOVA with a Greenhouse-Geisser correction determined that mean FMAS, AOUS and QOUS differed statistically significantly between time points, p-value < 0.0001. Post-hoc tests using the Bonferroni correction revealed that FMAS, AOUS and QOUS had been increased statistically significantly from pre to 1 month and then from 1 month to 3 months [Table/Fig-5,6].
Comparison of scores at 0 week, 1 month and 3 months in the study group.
| Study group | 0 week | 1 month | 3 months | p-value | pre vs 1 month | pre vs 3 months | 1 month vs 3 months |
|---|
| FMAS |
| Mean ± SD | 34.67 ± 3.55 | 48.1 ± 5.42 | 50.57 ± 4.97 | <.0001 | <.0001 | <.0001 | <.0001 |
| Median | 35.5 | 49 | 52 |
| Min-Max | 28-40 | 30-56 | 33-57 |
| AOUS |
| Mean ± SD | 11.5 ± 2.15 | 18.07 ± 2.5 | 19.7 ± 2.64 | <.0001 | <.0001 | <.0001 | <.0001 |
| Median | 12 | 18 | 19 |
| Min-Max | 8-15 | 13-23 | 15-25 |
| QOUS |
| Mean ± SD | 10.43 ± 2.06 | 16.8 ± 2.73 | 18.2 ± 2.67 | <.0001 | <.0001 | <.0001 | <.0001 |
| Median | 10.5 | 16.5 | 18.5 |
| Min-Max | 7-15 | 11-22 | 14-23 |
Comparison of scores at 0 week, 1 month and 3 months in the control group.
| Control group | 0 week | 1 month | 3 months | p-value | pre vs 1 month | pre vs 3 months | 1 month vs 3 months |
|---|
| FMAS |
| Mean ± SD | 34.7 ± 3.19 | 45.4 ± 3.42 | 46.93 ± 3.41 | <.0001 | <.0001 | <.0001 | <.0001 |
| Median | 35 | 46 | 47.5 |
| Min-Max | 28-40 | 40-51 | 41-52 |
| AOUS |
| Mean ± SD | 11.4 ± 2.09 | 16.87 ± 2.52 | 18.03 ± 2.77 | <.0001 | <.0001 | <.0001 | <.0001 |
| Median | 11.5 | 17 | 18 |
| Min-Max | 8-15 | 13-21 | 11-23 |
| QOUS |
| Mean ± SD | 10.2 ± 1.95 | 15.5 ± 2.11 | 16.73 ± 2.46 | <.0001 | <.0001 | <.0001 | <.0001 |
| Median | 10 | 15.5 | 17 |
| Min-Max | 7-14 | 12-20 | 12-21 |
At baseline there were no significant differences in FMAS, AOUS and QOUS between the two groups (p-values 0.9696, 0.8557, 0.6546 respectively). Post-hoc analysis revealed that compared to conventional rehabilitation group, study group showed significantly better scores at 1 month {FMA1 (p-value <.0001, es 0.2870), AOU1 (p-value 0.0007, es 0.1830), QOU1(p-value 0.0015, es 0.1640)} and 3 months {FMA3(p-value <.0001, es 0.4240), AOU3 (p-value 0.0003, es 0.2030), QOU3 (p-value 0.0008, es 0.1790)} as shown by the [Table/Fig-7]. Thus, after one month of therapy, the patients doing mCIMT along with conventional rehabilitation programme exhibited greater benefits as compared to patients doing conventional therapy alone and this was maintained at 3 months of follow-up. There were no adverse events during the study.
Comparison of scores between the two groups.
| Study group | Control group | p- value | p-value after adjusting for baseline using ANCOVA | Effect size |
|---|
| FMAS1 |
| Mean ± SD | 48.1 ± 5.42 | 45.4 ± 3.42 | 0.0247 | <.0001 | 0.2870 |
| Median | 49 | 46 |
| Min-Max | 30-56 | 40-51 |
| AOUS1 |
| Mean ± SD | 18.07 ± 2.5 | 16.87 ± 2.52 | 0.0691 | 0.0007 | 0.1830 |
| Median | 18 | 17 |
| Min-Max | 13-23 | 13-21 |
| QOUS1 |
| Mean ± SD | 16.8 ± 2.73 | 15.5 ± 2.11 | 0.0438 | 0.0015 | 0.1640 |
| Median | 16.5 | 15.5 |
| Min-Max | 11-22 | 12-20 |
| FMAS3 |
| Mean ± SD | 50.57 ± 4.97 | 46.93 ± 3.41 | 0.0017 | <.0001 | 0.4240 |
| Median | 52 | 47.5 |
| Min-Max | 33-57 | 41-52 |
| AOUS3 |
| Mean ± SD | 19.7 ± 2.64 | 18.03 ± 2.77 | 0.0204 | 0.0003 | 0.2030 |
| Median | 19 | 18 |
| Min-Max | 15-25 | 11-23 |
| QOUS3 |
| Mean ± SD | 18.2 ± 2.67 | 16.73 ± 2.46 | 0.0310 | 0.0008 | 0.1790 |
| Median | 18.5 | 17 |
| Min-Max | 14-23 | 12-21 |
After applying ANCOVA taking baseline as covariate there is significant difference in values of FMAS, AOUS and QOUS between study group and control group at 1 month and 3 months.
Discussion
Functional improvement of paretic upper limb is one of the primary goals of rehabilitation of hemiplegic patients. Various rehabilitation interventions have been investigated and applied with varying degrees success in achieving this goal [3,4]. CIMT and Modified CIMT have emerged as promising tool of rehabilitation of paretic upper limb [9,23,24]. Neuroimaging studies have demonstrated that the brain undergoes neuroplastic changes in function and structure in stroke patients who participate in CIMT and mCIMT [10,11]. Many cortical areas like primary motor cortex, dorsal premotor cortex and supplementary motor area shows increased electrical and metabolic neuronal activity during CIMT and mCIMT [28]. In this prospective study we compared mCIMT with conventional rehabilitation programme in the management of paretic upper limb.
FMA score showed significantly better outcome at one month and three months in mCIMT group as compared to conventional therapy group. Similar findings were reported earlier in study done by Page SJ et al., [14]. The substantial improvement in the abnormal movement patterns, reflected by FMA scores, in the mCIMT group suggested that mCIMT reversed impairments rather than simply helping patients to adapt to residual impairments.
Before intervention MAL scores were low for all the subjects. After the intervention, subjects in the study group showed significantly better scores on both the AOU Scores and QOU Scores of the MAL scale than those in the control group, suggesting the increased use of affected hand for ADL activities both qualitatively and quantitatively. These findings on AOU Scores and QOU Scores were consistent with previous studies done by Wu CY et al., and Page et al., [13,14]. This improvement in the MAL scores after intervention implies that the learned non-use phenomenon observed in the patients could be overcome by mCIMT [6].
No significant adverse events occurred during the treatment period and no major compliance issue was faced during our study.
In previous studies on mCIMT, therapy time has varied from 30 minutes per day for 3 days per week over duration of 10 weeks to 3 hours per day for 3 days per week over duration of 2 weeks and constraint time was either 5 hours or 6 hours [17–23]. In our study we checked the efficacy of mCIMT protocol of total treatment duration of 4 weeks with a constraint session of 5 hours per day for 5 days a week and a therapy session of 3 hours per day for 3 days a week. Results of our study have demonstrated that this protocol of mCIMT was associated with greater improvement in motor control and daily functioning than conventional rehabilitation methods. These results were consistent with the previous studies [23,29,30].
Limitation
However, this study could not examine the long term efficacy of mCIMT due to limited time and resources. Therefore it is important that future studies examine benefits of mCIMT in a larger sample of stroke patients and for a longer period of time.
Conclusion
The result of our study provides experimental data addressing the improvement in hand function of stroke patients following mCIMT in terms of motor recovery and functional outcome. In our study all measured outcomes were improved significantly with a mCIMT protocol of treatment time 3 hours per day for 3 days per week and constraint time of 5 hours per day for 5 days per week over a total duration of 4 weeks. Hence, it can be concluded that our mCIMT protocol is a convenient and acceptable design to both patients and care giver.
Independent t test applied except minimum active extension at wrist and MCP criteria for which Mann Whitney U test applied.
After applying ANCOVA taking baseline as covariate there is significant difference in values of FMAS, AOUS and QOUS between study group and control group at 1 month and 3 months.