Birth asphyxia is defined as a delay in establishing spontaneous breathing/crying immediately after birth of the newborn [1]. This results in impaired gas exchange, hypoxia and metabolic acidosis [2]. But it is probably better to use the term perinatal asphyxia since asphyxia may occur in utero, at birth or in the postnatal period. Hypoxic Ischemic Encephalopathy (HIE) is the term commonly used to describe the neurological syndrome that occur following perinatal asphyxia. It is usually caused by severe birth asphyxia with secondary cerebral ischemia [3]. It is an important cause of permanent damage to the Central Nervous System (CNS) which may result in neonatal death or manifest later as cerebral palsy or mental deficit [4]. Throughout the world each year, an estimated 23% of the 4 million neonatal deaths and 8% of all deaths in <5 years of age are associated with signs of asphyxia at birth [5]. Most of these infants are born in developing nations, and more than 50% of the deaths occur at home, where majority of these infants are born [6]. A 15%-20% of HIE cases die during the neonatal period and 30% of those who survive, suffer from neurodevelopment disorders [5,7,8]. In less developed countries perinatal asphyxia remains a major cause of death and disability. The pattern of risk factors, the nature of sequelae and the options and priorities for intervention (both preventive and therapeutic) are significantly different than in the industrialized countries [9]. The HIE score (Thompson score) is a clinical tool comprising of a set of clinical signs associated with CNS dysfunction. It is used to assess status of a child following birth asphyxia [10–13]. In the scoring system, a score of 0 is normal and the maximum score is 22 which signifies the worst possible status of HIE. Infants with score 1–10 are considered to have mild HIE, 11–14 have moderate HIE and 15–22 are considered to have severe HIE. In normothermic infants, a maximum score of > 10 during the first 7 days of life predicts an abnormal outcome with 100% sensitivity and 61% specificity [14]. This study was done to assess the role of cord arterial blood gas analysis at birth and serial Thompson score (HIE score) in predicting the early neonatal outcome in post asphyxiated term neonates.
Materials and Methods
The study was conducted in Department of Paediatrics, in NICU, Hindu Rao Hospital, New Delhi from May 2014 to February. 2015. This study was a prospective cross-sectional study. During this period, a total of 145 post asphyxiated term neonates born in labour room/obstetric operation theatre were recruited [Table/Fig-1]. An informed consent was taken from all the parents. The protocol was approved by the institutional ethical committee.
Selection of data by inclusion and exclusion criteria.
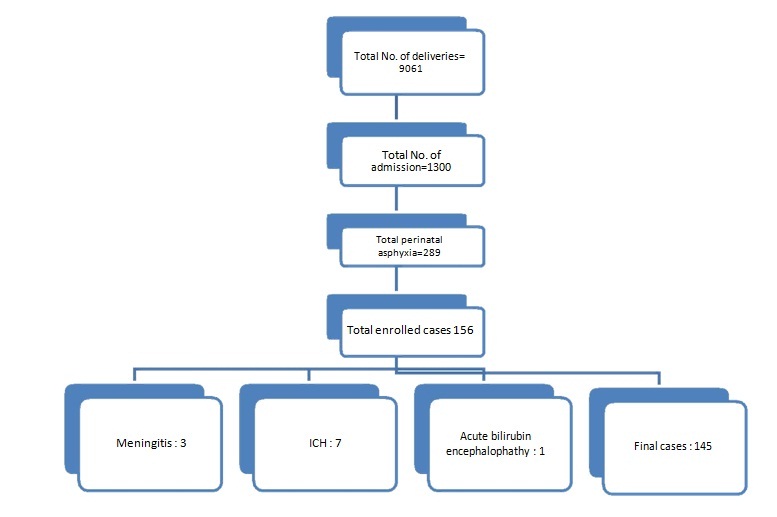
Inclusion Criteria: Full-term babies with low-Apgar score i.e., 1 min score of ≤7. (NNPD) [15].
Exclusion Criteria: Preterm babies, Respiratory depression due to intracranial bleed, Neonates with major congenital malformations of CVS, CNS, Respiratory system or dysmorphic babies, Severe hyperbilirubinemia bordering on kernicterus, Cases with hypoglycaemia or meningitis as cause of encephalopathy.
Criteria to define HIE: HIE characterized by Thompson score above 5 one hour after birth [16]. The score is derived from nine aspects of the neurological examination of infants with HIE, shown in [Table/Fig-2].
The Thompson HIE score [14].
| ScoreSign | 0 | 1 | 2 | 3 |
|---|
| Tone | Normal | Hyper | Hypo | Flaccid |
| LOC | Normal | Hyperalert, stare | Lethargic | Comatose |
| Fits | None | < 3/day | >2/day | |
| Posture | Normal | Fisting, cycling | Strong distal flexion | Decerebrate |
| Moro | Normal | Partial | Absent | |
| Grasp | Normal | Poor | Absent | |
| Suck | Normal | Poor | Absent ± bites | |
| Respiration | Normal | Hyperventilation | Brief apnea | IPPV (apnea) |
| Fontanelle | Normal | Full, not tense | Tense | |
Cord arterial blood gas analysis was done at birth (pH, p CO2, Bicarbonate, base deficit). Arterial cord blood samples were collected from double clamped umbilical cord, anaerobically, using heparinized disposable syringes (2ml syringe washed by 1000IU/ ml heparin) and the samples were analysed by Blood Gas Analyser –Bayer, Germany. It directly measures pH, pCO2, bicarbonate, base deficit. All infants with severe post-asphyxial HIE had evidence of dysfunction of at least one organ/system in addition to the central nervous system. This conforms with the criteria of the American College of Obstetricians and Gynaecologists [17] and Society of Obstetricians and Gynaecologists of Canada [18].
Criteria to define outcome: Outcome measures were grouped as: Normal, Morbidity, Neonatal encephalopathy with seizures, Neonatal encephalopathy with seizures requiring anticonvulsant medication, Neonatal encephalopathy with seizures requiring medication, within 24 hours of birth, Apgar score <7 at 5 minutes and admission to the neonatal unit and death within 4 weeks [19].
Statistical Analysis
SPSS 17.0 software has been used for data analysis. The data were expressed in terms of Means, Standard Deviation and Proportion, followed by comparison between groups through chi-square test or Fisher’ p-value of less than 0.05 was considered as statistically significant.
Results
Apgar score of <=3 at 1minute was found in 58 babies (40%) and 87 babies (60%) had a score of 4-6 at 1 minute.
Mild Thompson score on day 1,3,7 were, 96 (66.2%), 119 (82.06%), 125 (86.20%), moderate Thompson score on day 1,3, 7 were 13 (8.9%), 6 (4.13%), 2 (1.37%) and severe Thompson score on day 1, 3, 7 were 36 (24.8%), 13 (8.96%), 7 (4.82%) respectively as given in [Table/Fig-3]. Total 11 patients died within 7 days among them 7 patients died within 3 days. There was clinical improvement among HIE patients as indicated by serial Thompson score done on day 1, 3 and 7. Out of 145 patients, cord gas acidosis was found in 54 patients as given in [Table/Fig-4], there is statistically significant correlation between morbidity and day 1 Thompson score (p-value 0.024). There is statistically significant correlation between mortality and day 1 Thompson score (p-value 0.001). There is a statistically significant relation between duration of hospital stay and HIE grading (p=0.008) with maximum duration of stay in severe HIE cases (mean value 7.64 days) total 62 (42.8%) patients had seizures. The type of seizures and management is as given in [Table/Fig-5,6 and 7].
Grading and evolution of Thompson score at Day 1, 3 & 7 (improvement/survival rate).
| Thompson score | Day 1 | Day 3 | Day 7 |
|---|
| n = 145 | n = 145 | n = 145 |
|---|
| Mild (1 - 10) | 96 | 119 | 125 |
| Moderate (11 - 14) | 13 | 6 | 2 |
| Severe (15+) | 36 | 13 | 7 |
Correlation between 5 minute Apgar score and cord gas acidosis.
| Apgar score at 5 min | Cord gas acidosis | p |
|---|
| Yes | No | Total |
|---|
| n = 145 (%) | n = 145 (%) | n = 145 (%) |
|---|
| <=3 | 16 (29.6) | 1 (1.1) | 17 (11.7) | <0.001 |
| >3 | 38 (70.4) | 90 (98.9) | 128 (88.3) |
| Total | 54(100) | 91(100) | 145(100) | |
Co-relation between HIE score on day 1 and neonatal convulsion.
| Seizures | HIE | p |
|---|
| M ild | M oderate | Severe | Total |
|---|
| n = 145 (%) | n = 145 (%) | n = 145 (%) | n = 145 (%) |
|---|
| No | 67(69.8) | 1(7.7) | 15(41.7) | 83(57.2) | <0.001 |
|---|
| Yes | 29(30.2) | 12(92.3) | 21(58.3) | 62(42.8) |
| Total | 96(100) | 13(100) | 36(100) | 145(100) | |
Co-relation between HIE score on day 1 and neonatal convulsion.
| Types of seizures | Subtle | Tonic | Multifocal | Total |
|---|
| n = 62 (%) | n = 62 (%) | n = 62 (%) |
|---|
| No. (%) | 25(40.3%) | 16(25.8%) | 21(39.9%) | 62(100%) |
Babies who required anticonvulsants.
| Phenob-arbitone loading dose | + Pheno-barbitone maintenance dose | + phenytoin Loading dose | + phenytoin Maintenance Dose | + Midazolam infusion | Total |
|---|
| n = 62 (%) | n = 62 (%) | n = 62 (%) | n = 62 (%) | n = 62 (%) | |
|---|
| No. of babies | 50 (80.6) | 5 (8%) | 3 (4.83) | 2 (3.22) | 2 (3.22) | 62 |
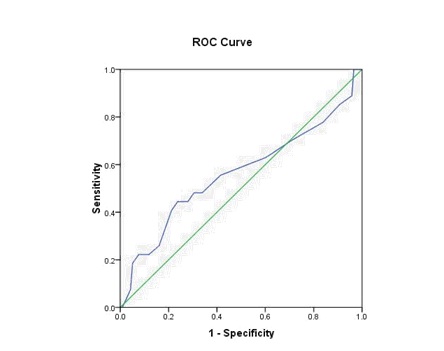
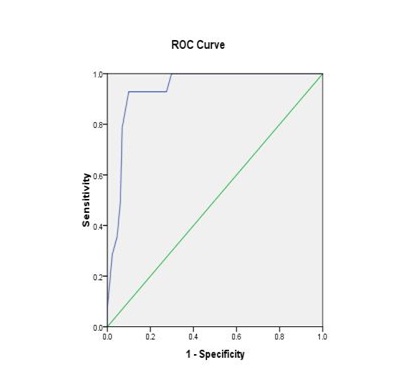
In 145 cases as depicted in [Table/Fig-8,9], ROC curves were plotted for Thompson score day 1 and morbidity and Thompson score day1 and mortality. For the mortality curve, AUC (Area under Curve) was 0.936 which was highly significant (p=0.001). There was a tendency for better predictive capacity of Thompson score in regard to morality (Sensitivity 93%, Specificity 90%, Positive Predictive Value (PPV) 50% and Negative Predictive Value (NPV) 99%). For the morbidity curve, Area Under Curve (AUC) was 0.561 which was not significant (p=0.324), Sensitivity was 63%, Specificity was 40%, PPV was 19% and NPV was 82%.
ROC curve (Morbidity with Thompson score).
ROC curve (Mortality with Thompson score).
Discussion
The present study was carried out on 145 newborns with perinatal asphyxia admitted to NICU, department of pediatrics Hindu Rao Hospital Delhi. For delivery room resuscitation, the standard NRP AAP 2010 guidelines were followed and cord arterial blood gas analysis was done in each patient. The cases were further divided into mild, moderate and severe HIE, according to Thompson score.
In present study 57 (39.3%) patients had maternal risk factor. The most common maternal risk factor was those mother who didn’t register ANC (Anti Natal Checkup) as given for in [Table/Fig-10] [20–22].
Comparative study of relationship between maternal risk factor and perinatal asphyxia [20–22].
| Maternal risk factor | Maternal age35 yrs≥ | No/<3 Antenatal Visit | PIH (Pregnancy induced hypertension | BOH (Bad Obstetric History) | Gestational Diabetes | Total | No maternal risk factor |
|---|
| No. of babies n=145 (%) | 15 (10.3) | 29 (20) | 16 (11) | 8 (5.5) | 7 (4.8) | 57 (39.3) | 88 (60.7) |
| Author | Dongol S et al., [20] (9.8%), Mundhra R et al., [21] (23.6%) | Dongol S et al., [20] (15.68%) | Mundhra R et al., [21] (16.97%), Kerkhofs C et al., [22] (9.4%). | ------ | Kerkhofs C et al., [22] (5.7%). | ------ | |
MSL (Meconiu m Stained Liquor) was the most common intrapartum risk factor seen in 54 (37.2%) cases which was in accordance with Dongol S et al., [20] (37.2%), but higher than Padayachee N et al., [23] (10.9%) and lesser than Shrestha M et al., [24] (65%). Post term as an intrapartum risk factor was present in 10 (6.9%). Our finding is similar to Mundhra R et al., [21] (6.06%), Qureshi AM et al., [25] (6%). We found prolonged labour in 8 (5.5%) which was lesser than Qureshi AM et al., [25] (9.3%).
There is strong association of cord gas acidosis (pH <7) and severity of HIE in the present study as given in [Table/Fig-11] [26–29]. In normothermic infants, Thompson score of >10 during the first 7 days of life predicts an abnormal outcome with 100% sensitivity and 61% specificity [14].
Association of cord gas acidosis and HIE in prediction of outcomes [26–29].
| Study | Findings |
|---|
| Malin et al., [26] | Low arterial pH was strongly associated with neonatal mortality and long term adverse outcomes. |
| Haddad et al., [27] | Arterial cord pH <7 was associated HIE and death within neonatal period. |
| Ghosh et al., [28] | Arterial cord pH ≤7.15 was associated with Hypoxic ischaemic encephalopathy and death during neonatal period. |
| Graham EM et al., [29] | 109 | 33 (30.3%) HIE 27 (24.8%) seizures 5 (4.6%) deaths |
| Present study | 54 | 17.7% had mild HIE, 53.8% had moderate HIE and 83.3% had severe HIE |
Limitation
Long term neurodevelopmental outcome was not assessed in our study and outcome prediction could better be assessed with the help of neuroimaging.
Conclusion
Thompson score allows a very precise description of infants by assigning a numeric score rather than ‘mild’, ‘moderate’ or ‘severe. Inter-rater reliability is very good with a kappa coefficient of 0.8 and there is no requirement of Electroencephalogram which is beneficial in resource limited country like ours. Acid-base analysis in the first hour of life suggests that a threshold level of the pH and base deficit may indicate the presence of intrapartum hypoxia more specifically and objectively than a low Apgar score or an abnormal Cardiotocography.