Transthoracic Biopsy Causes Massive Subcutaneous Emphysema in a Low Risk Patient
Fikri Selcuk Simsek1, Yusuf Dag2
1 Medicine Faculty, Department of Nuclear Medicine, Firat University, Elazig, Turkey.
2 Faculty, Department of Nuclear Medicine, Balikesir State Hospital, Balikesir, Turkey.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Fikri Selcuk Simsek, Medicine Faculty, Department of Nuclear Medicine, Firat University, Elazig-23200, Turkey.
E-mail: fselcuksimsek@gmail.com
Subcutaneous Emphysema (SE) can be defined as air leakage under skin from the respiratory or gastrointestinal system. It is frequently accompanied by pneumomediastinum. Thoracentesis, image-guided lung biopsies, pulmonary diseases and therapies resulting in necrosis can cause this pathology. The risk of pneumothorax and SE increased with the distance of the lesion to the pleura, and small size of the lesion. Although, our patient had low risk for SE, there were minimal pneumothoraces and massive SE. We consider that tumour necrosis and subcutaneous tissue may be related via transthoracic biopsy and this leads to massive SE.
Emphysema, Lung cancer, Pneumothoraces
Case Report
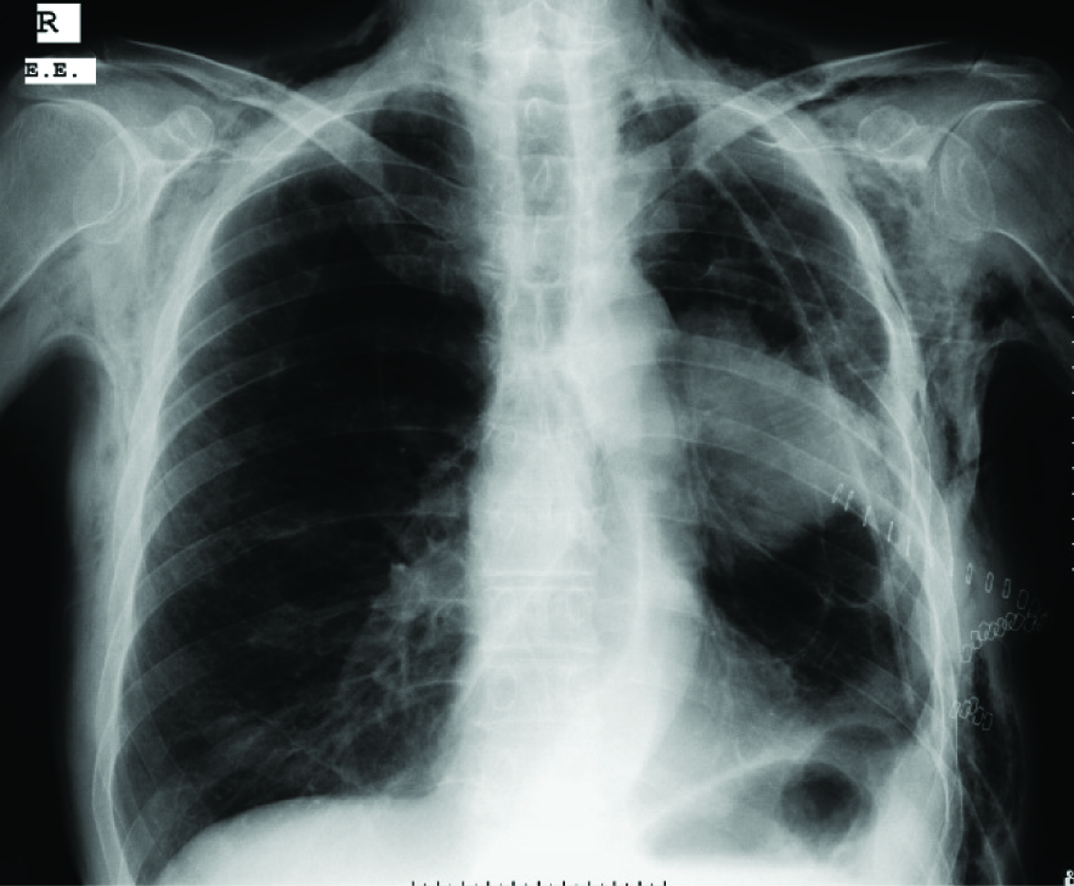
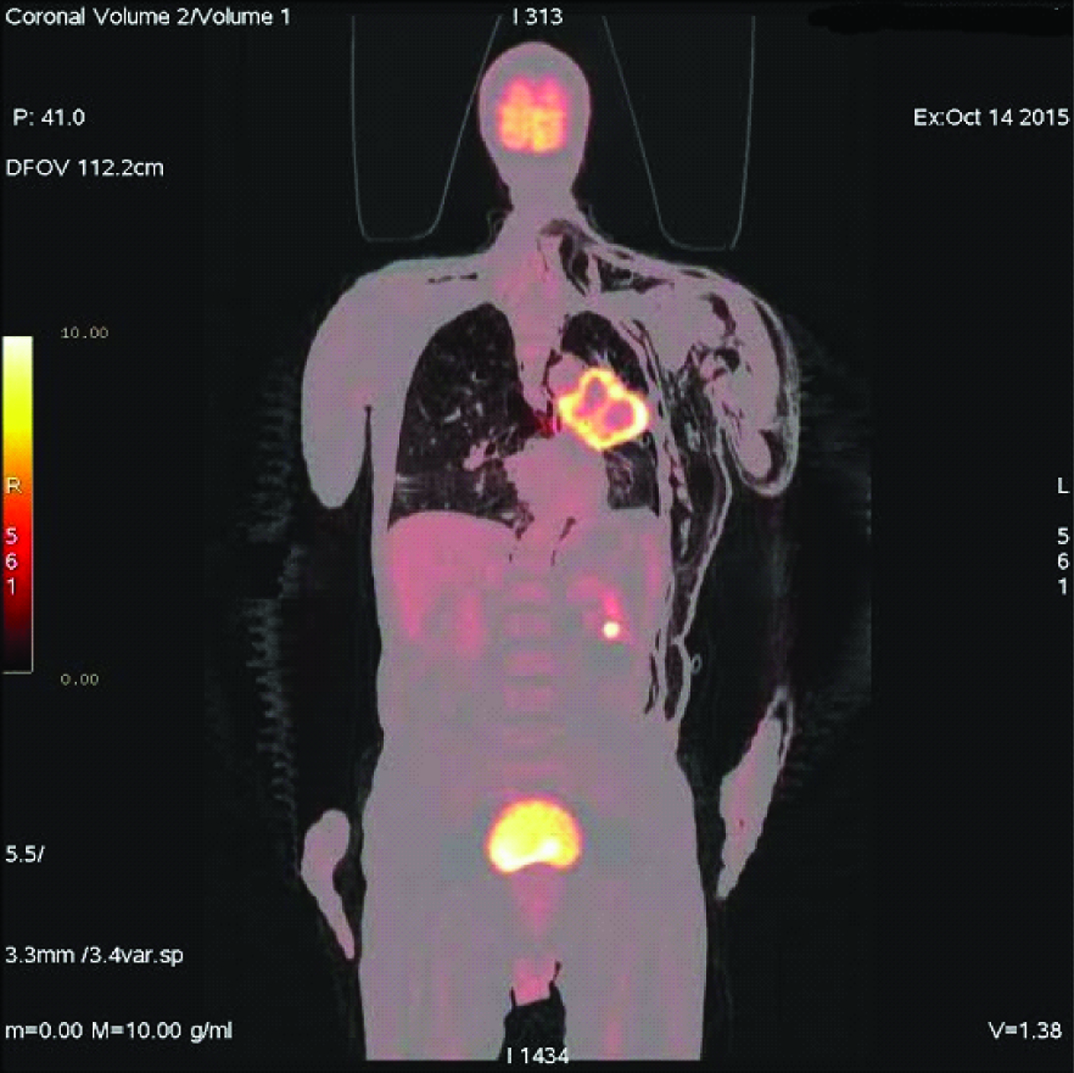
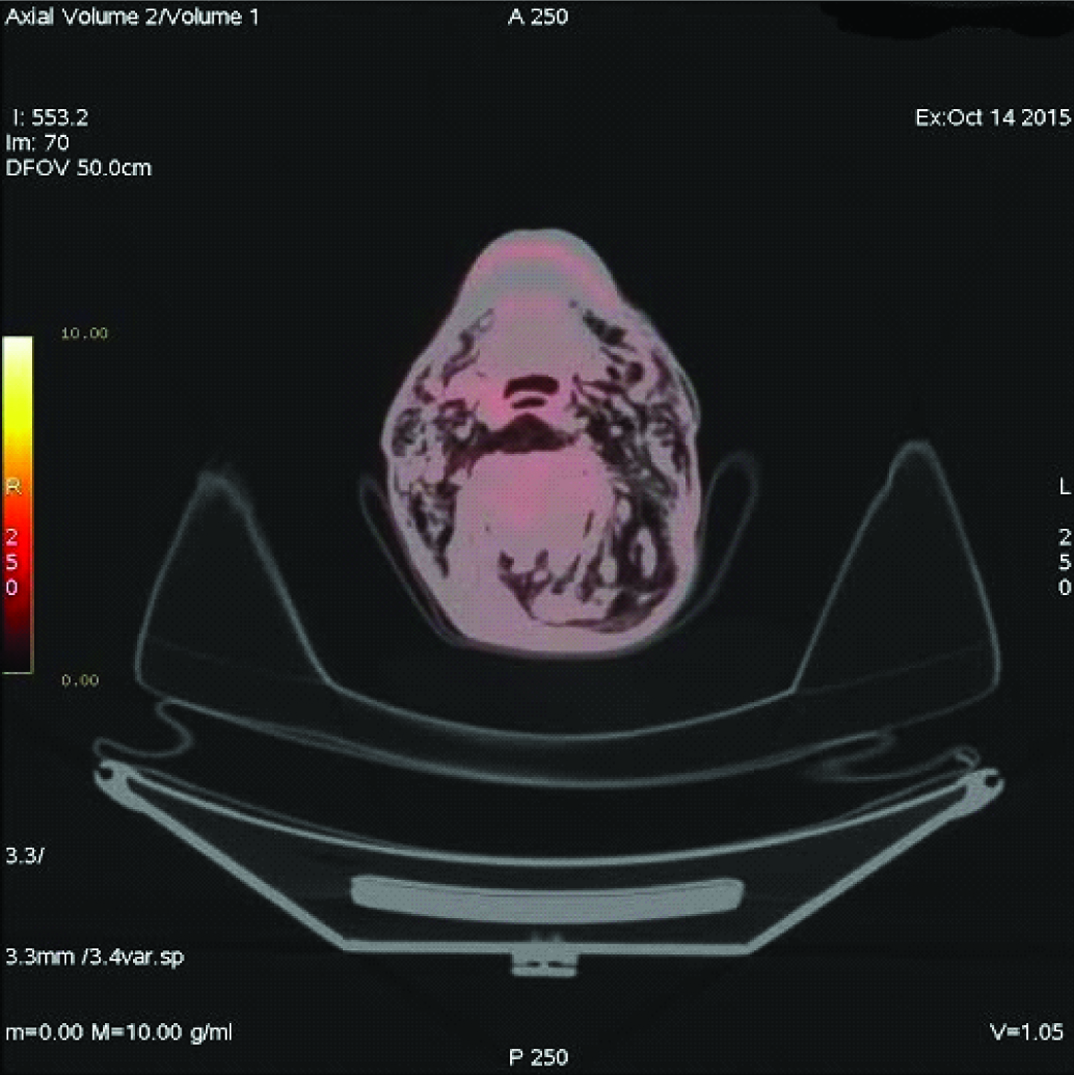
A 69-year-old male patient complained of dyspnoea, cough, and fatigue since one month. On physical examination respiratory rate was 26/minute, blood pressure 140/85mmHg and pulse rate 82/minute. Percussion determined submatity in the middle lobe of the left hemi-thorax on auscultation. In the chest radiography [Table/Fig-1], there was a space-occupying lesion in the left hilus. Spirometry results were Force Expiratory Volume 1= 69, and Force Expiratory Volume 1/Force Vital Capacity = 95. A non-contrasted CT thorax was performed one day later, it was observed that a mass with 8.9 cm diameter was located in the left hilus of the lung, and a minimal pneumothorax was determined at the inferolateral part of left hemithorax. The patient refused bronchoscopic biopsy so transthoracic biopsy was performed. After transthoracic biopsy, broncoscopy was performed. The histopathologic report confirmed it as squamous cell carcinoma. Four days after the transthoracic biopsy, swelling started in the posterior part of the body and expanded progressively. After seven days of biopsy, Positron Emission Tomography/Computed Tomography imaging was done for staging of primary mass, which detected necrosis in the middle of tumour [Table/Fig-2]. It also revealed, minimal pneumothorax in the left hemithoraces and massive subcutaneous emphysema extended in bilateral neck region [Table/Fig-3], left side of the body and it sprawled up to L4 vertebral level in the left side. So, final diagnosis was lung cancer and transthoracic biopsy related massive SE. Patient refused for the lung cancer treatment and died three months after the diagnosis.
Chest radiography showing space-occupying lesion in the left lung (hilus).
Necrosis in the middle of primary lung carcinoma in left lung.
Emphysema in bilateral subcutaneous neck tissue.
Discussion
PET means using positron emitting radiopharmaceuticals for imaging purposes. In PET studies, the most commonly used radiopharmaceutical is 2-fluoro-2-deoxy-D-glucose (18 F-FDG) and is based on the principle of high glucose consumption by malignant cells. Mostly CT component is added to PET devices for attenuation correction and anatomic localization. PET/CT is widely used for cancer staging including lung cancer [1,2].
Alveolo-pleural fistula is defined as an abnormal connection between the airways and pleural space [3]. Bronchocutaneous fistula is the direct connection between bronchial system and skin, and it is generally a rare complication of lung cancer [4]. Reports indicate that if there is a rupture in alveola, air leakage reaches to pulmonary interstitial tissue and then to perivascular space and pleura. If air passage exceeds pleural reabsorption capacity, SE may occur [5–7].
Thoracentesis, image-guided lung biopsies, pulmonary diseases and therapies resulting in necrosis can cause this pathology [3,4,8,9].
Aktas AY et al., suggested that 28.7% pneumothorax and 2.1% SE were developed after CT-guided biopsy procedure [10]. Meregildo et al., reported that after the percutaneous pulmonary biopsy, pneumothorax and SE may emanate [5]. Zheng A et al., reported concomitant massive SE in two patients with large pneumothorax [11]. The risk of pneumothorax proportionally increased with the distance of the lesion to the pleura [12], and the small size of the lesion [10,13]. In a study published in 2014, it was reported that tumour necrosis together with pleural adhesion might cause fistulization between the lung and subcutaneous tissue and finally result in massive subcutaneous emphysema and minimal pneumothorax [14]. Yalçınkaya et al., reported that cavity in tumoural tissue might cause SE without causing pneumothorax and pneumomediastinum [15]. There are some other studies in the literature reporting that there could be a relationship between tumour necrosis, pneumothoraces and SE [16–18].
In our case, it could have been expected that pneumothoraces and SE development risk was minimal because the lesion was large and near the pleura (approximately 10 mm distance) [10,12,13] however, it was not. We believe that tumour necrosis and subcutaneous tissue could be related with transthoracic biopsy and this led to massive SE and minimal pneumothoraces.
Conclusion
Massive SE and pneumothoraces may occur because of transthoracic biopsy if tumour has necrosis. These complications should be considered in patients who have necrotizing tumour even in low risk patients.
[1]. Metin M, Citak N, Sayar A, Pekcolaklar A, Melek H, Kök A, The role of extended cervical mediastinoscopy in staging of non-small cell lung cancer of the left lung and a comparison with integrated positron emission tomography and computed tomography does integrated positron emission tomography and computed tomography reduce the need for invasive Procedures?J Thorac Oncol 2011 6:1713-19. [Google Scholar]
[2]. Fischer B, Lassen U, Mortensen J, Larsen S, Loft A, Bertelsen A, Preoperative staging of lung cancer with combined PET-CTN Eng J Med 2009 361:32-9. [Google Scholar]
[3]. Abu-Hijleh M, Blundin M, Emergency Use of an Endobronchial One-Way Valve in the Management of Severe Air Leak and Massive Subcutaneous EmphysemaLung 2010 188:253-57. [Google Scholar]
[4]. Fraser A, Nolan RL, Malignant broncho subcutaneous fistula presenting as subcutaneous emphysemaJ Thorac Imaging 2002 17:319-21. [Google Scholar]
[5]. Alarcón-Meregildo KG, Polo-Romero FJ, Beato-Pérez JL, Tratamiento de enfisema subcutáneo severo por microdrenaje. A propósito de uncasoArch Bronconeumol 2014 50:47-8. [Google Scholar]
[6]. Beck PL, Heitman SJ, Mody CH, Simple construction of a subcutaneous catheter for treatment of severe subcutaneous emphysemaChest 2002 121:647-49. [Google Scholar]
[7]. Herlan DB, Landreneau RJ, Ferson PF, Massive spontaneous subcutaneous emphysema. Acute management with infraclavicular ‘blow holes’Chest 1992 102:503-05. [Google Scholar]
[8]. Powner DJ, Bierman MI, Thoracic and extrathoracic bronchial fistulasChest 1991 100:480-86. [Google Scholar]
[9]. Kashima M, Yamakado K, Takaki H, Kodama H, Yamada T, Uraki J, Complications after 1000 lung radiofrequency ablation sessions in 420 patients: A single center’s experiencesAJR Am J Roentgenol 2011 197:W576-80. [Google Scholar]
[10]. Aktas AY, Gozlek E, Yazkan R, Yilmaz O, Kayan M, Demirtas H, Transthoracic biopsy of lung masses: Non-technical factors affecting complication occurrenceThorac Cancer 2015 6:151-58. [Google Scholar]
[11]. Zheng A, Wang X, Yang X, Wang W, Huang G, Gai Y, Major Complications After Lung Microwave Ablation: A Single-Center experience on 204 SessionsAnn Thorac Surg 2014 98:243-48. [Google Scholar]
[12]. Yeow KM, Su IH, Pan KT, Tsay PK, Lui KW, Cheung YC, Risk factors of pneumothorax and bleeding: multivariate analysis of 660 CT-guided coaxial cutting needle lung biopsiesChest 2004 126:748-54. [Google Scholar]
[13]. Rizzo S, Preda L, Raimondi S, Meroni S, Belmonte M, Monfardini L, Risk factors for complications of CT-guided lung biopsiesRadiol Med 2011 116:548-63. [Google Scholar]
[14]. Yusuke K, Hiromasa Y, Takao H, Soh J, Toyooka S, Kanazawa S, Massive Subcutaneous and Mediastinal Emphysema with Little Pneumothorax Treated by Surgery after Pulmonary Radiofrequency AblationCardiovasc Intervent Radiol 2014 37:548-51. [Google Scholar]
[15]. Yalçinkaya S, Vural AH, Göncü MT, Ozyazicioglu AF, Cavitary lung cancer presenting as subcutaneous emphysema on the contralateral side cardio-Thoracic urgeryInteract Cardiovasc and Thorac Surg 2012 14:338-39. [Google Scholar]
[16]. Radvany MG, Allan PF, Frey WC, Banks KP, Malave D, Pulmonary radiofrequency ablation complicated by subcutaneous emphysema and pneumomediastinum treated with fibrin sealant injectionAJR Am J Roentgenol 2005 185:894-98. [Google Scholar]
[17]. Nishida T, Inoue K, Kawata Y, Izumi N, Nishiyama N, Kinoshita H, Percutaneous radiofrequency ablation of lung neoplasms: a minimally invasive strategy for inoperable patientsJ Am Coll Surg 2002 195:426-30. [Google Scholar]
[18]. Sakurai J, Hiraki T, Mukai T, Mimura H, Yasui K, Gobara H, Intractable pneumothorax due to bronchopleural fistula after radiofrequency ablation of lung tumoursJ Vasc Interv Radiol 2007 18:141-45. [Google Scholar]