Untreated bladder exstrophy in an adult is rare, as the defect is obvious and primary reconstruction is usually done in infancy. There are less than 90 reported cases of primary adenocarcinoma in an untreated bladder exstrophy in literature and only two such case reports from India. Of these, only one case was of signet-ring cell type of mucinous adenocarcinoma. Here we report the second case of signet-ring cell adenocarcinoma in a 63 year old male with untreated bladder exstrophy (oldest patient in literature), to highlight the extreme rarity, yet distinct possibility and challenges faced in surgical management of such cases.
Aged, Rectus abdominus, Mucinous adenocarcinoma
Case Report
A 63-year-old male came with chief complaints of serosanguinous discharge from a growth in lower abdomen and associated left flank pain since six months. He gave history suggestive of bladder exstrophy since birth for which he had not sought any treatment.
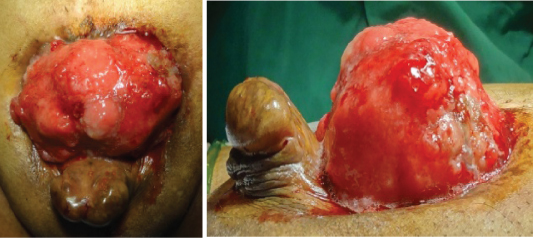
On examination, there was a 5x6 cm sized non-tender, exophytic mass involving nearly entire exposed surface of bladder which bled on touch. Bilateral ureteric orifices were not seen separately from the mass. Penile epispadias was also present [Table/Fig-1,2]. Scrotum was underdeveloped with small bilateral testes (both approximately 10-12 cc). The pubic bones were widely separated with diverication of recti. His haemoglobin was 11 g/dl, random blood sugar was 108 mg/dl, serum bilirubin was 0.6 mg/dl, serum AST 35 U, ALP 140 IU/l, and serum creatinine was 2 mg/dl. Ultrasonography showed left moderate hydronephrosis with left hydroureter. Left sided percutaneous nephrostomy was done which brought creatinine levels to 1 mg/dl in seven days.
The exstrophied bladder with tumor.
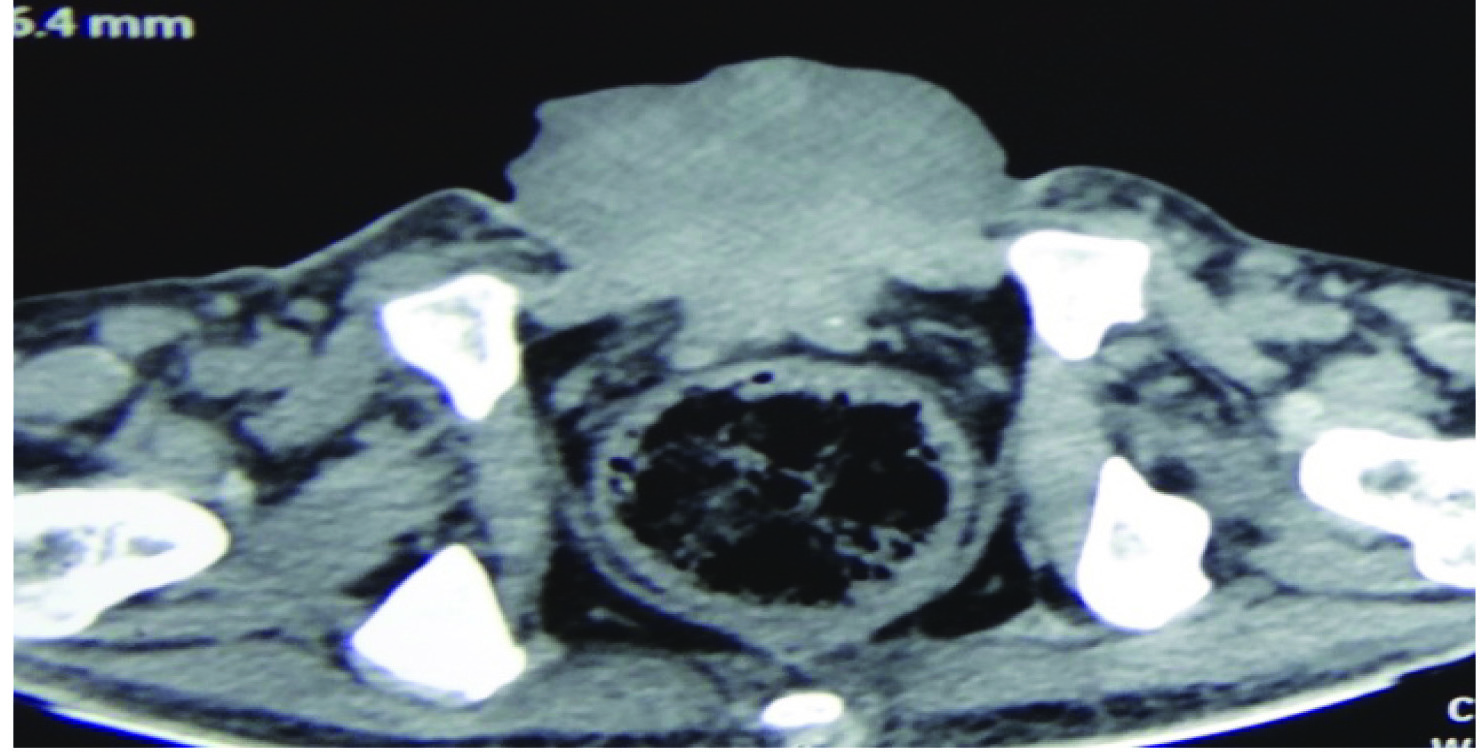
Contrast Enhanced Computed Tomography (CECT) abdomen was then done once creatinine level was 1 mg/dl. Computed Tomography (CT) scan was consistent with above findings [Table/Fig-3]. Chest X-ray was normal. Biopsy from the mass was suggestive of mucinous adenocarcinoma of bladder.
CT scan showing mass arising from exstrophied bladder.
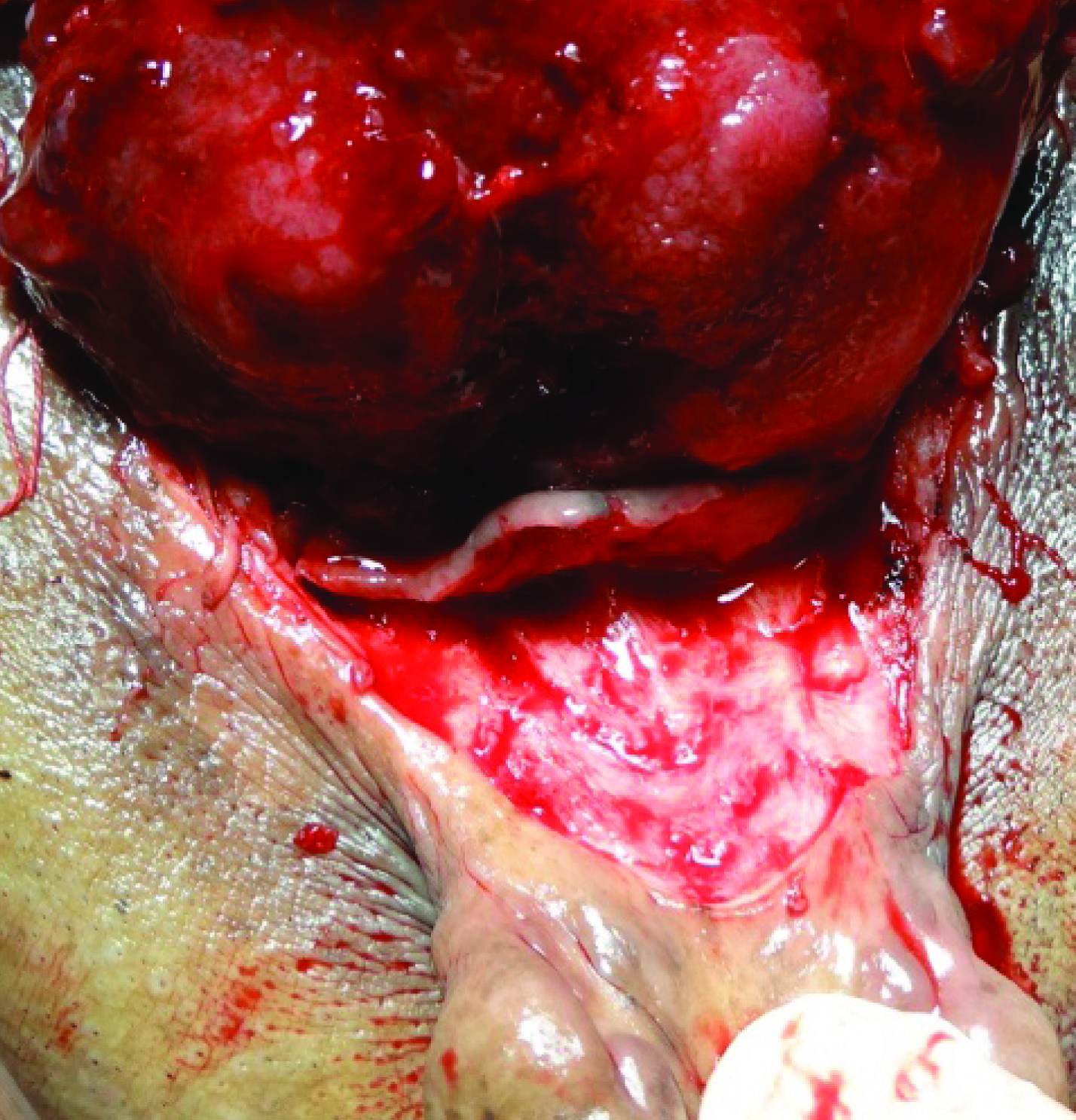
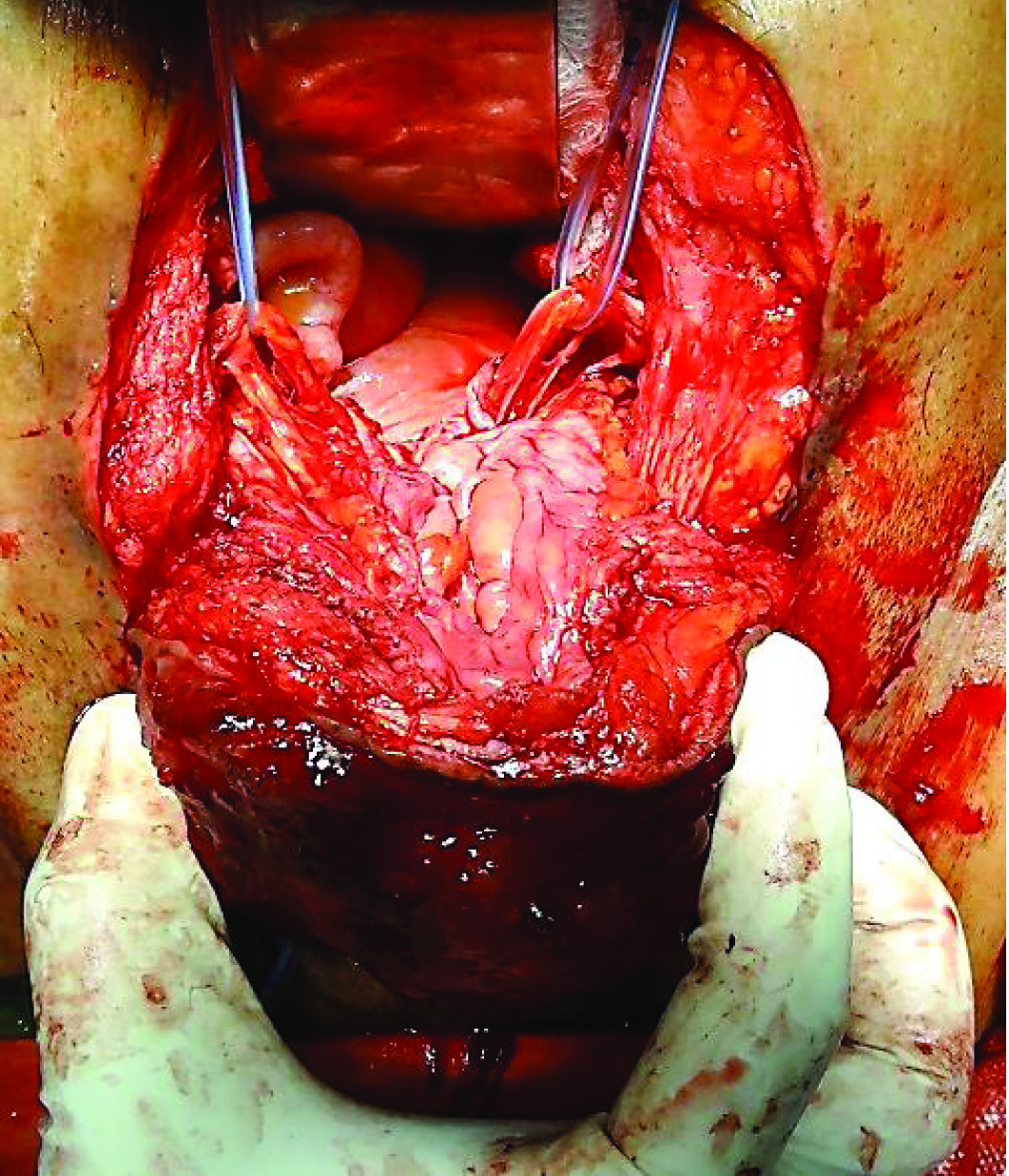
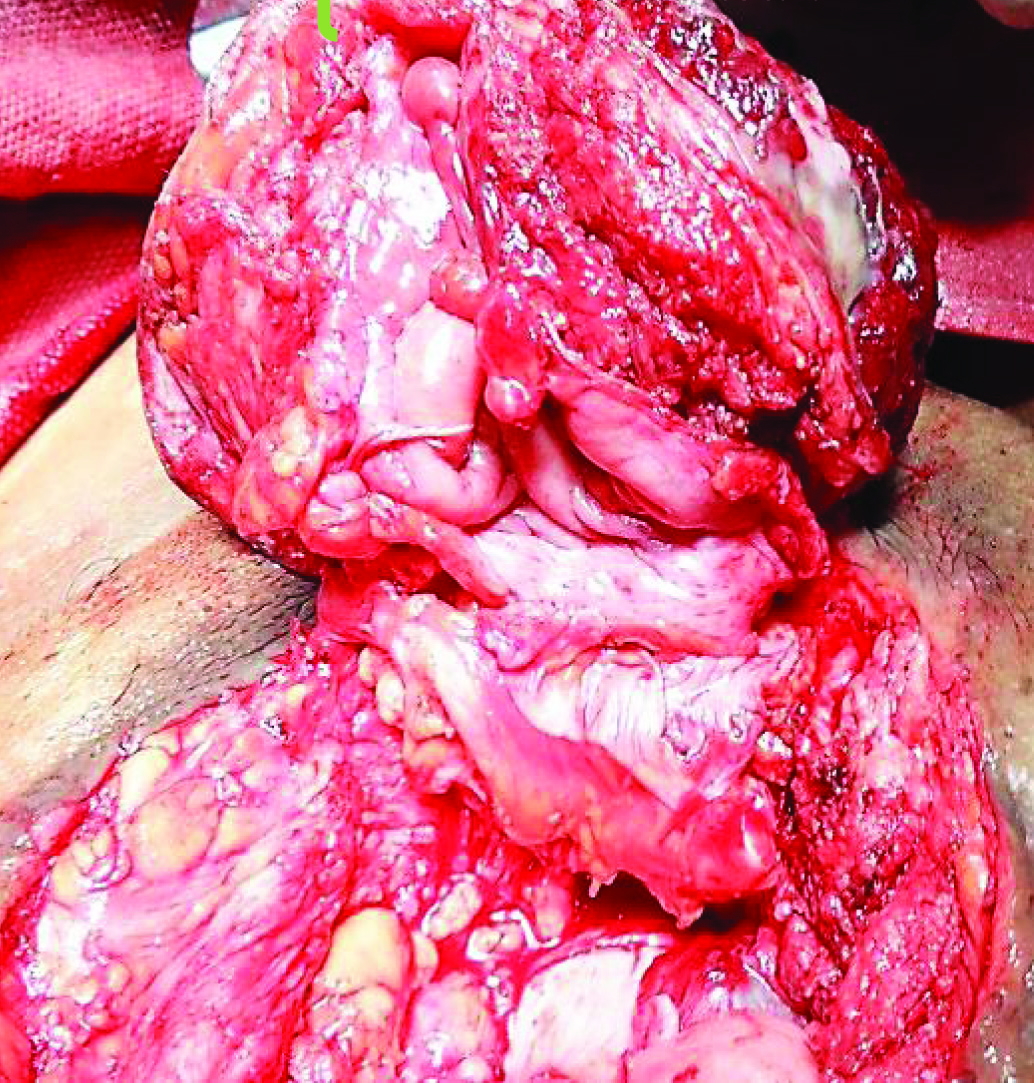
Patient eventually underwent radical cystectomy, lymph node excision and wide local excision (1 cm margin of resection) of skin with ileal conduit [Table/Fig-4,5,6 and 7].
Lower end of tumor being separated from skin, with 1 cm margin of resection.
Tumor separated from surrounding abdominal wall cranially and laterally.
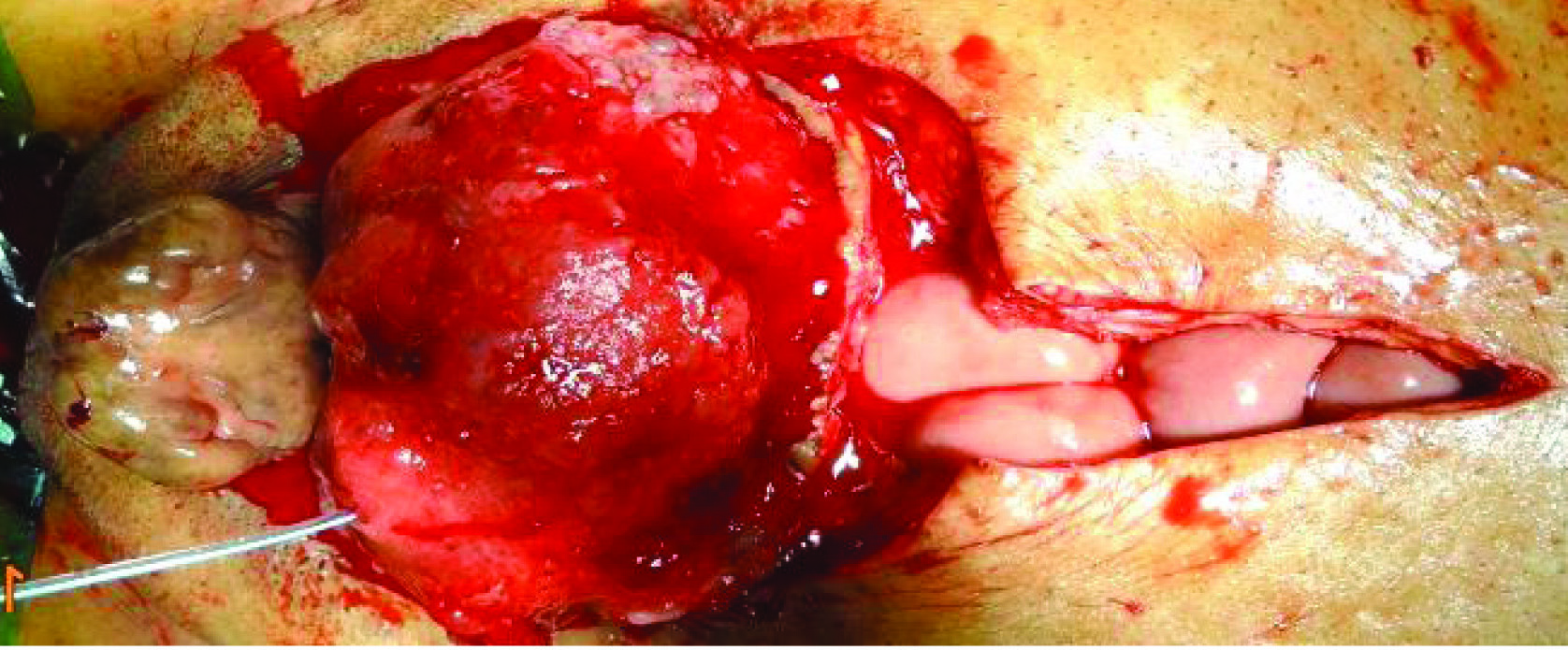
Bilateral ureters identified and safeguarded.
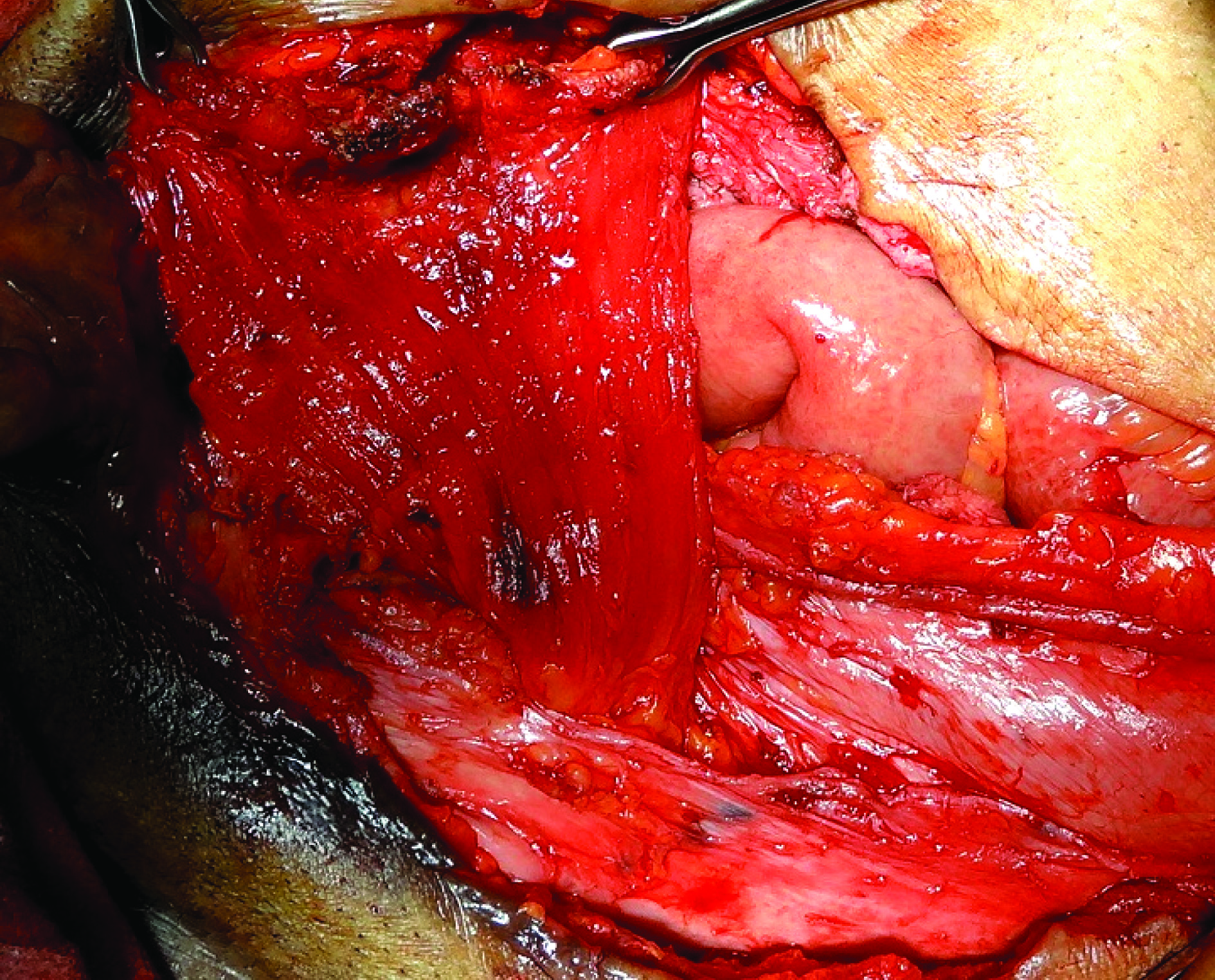
Abdominal wall defect was ten by nine cm. Primary closure with rectus abdominis approximation was not possible due to diverication. So, it was closed with rectus abdominis rotation flap [Table/Fig-8] based on inferior epigastric artery. Skin was closed primarily over it.
Cross Rectus Abdominis flap
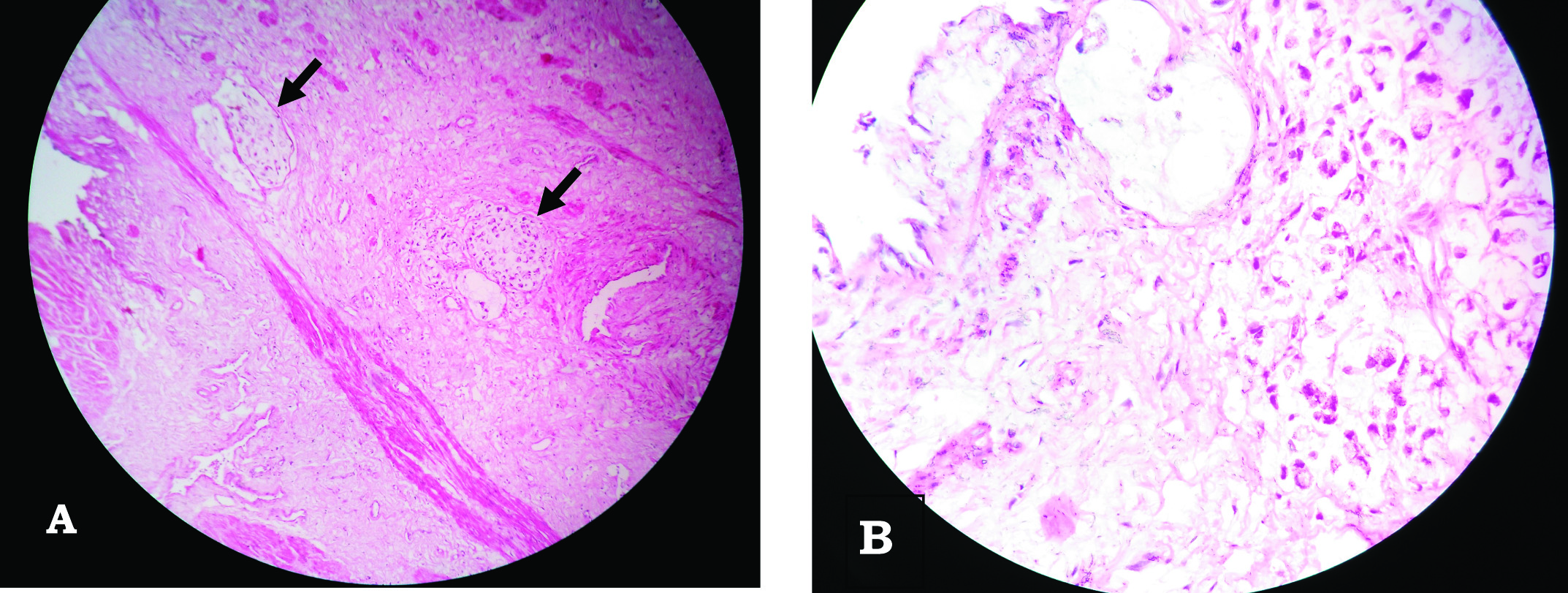
Postoperative convalescence was uneventful. Histopathology examination [Table/Fig-9] revealed signet-ring cell type adenocarcinoma of bladder with all margins free of tumor and no lymph node involvement. The ureteric margins were also free of tumor. No adjuvant therapy was given. Patient has been followed up for one year and has no complaints.
Histopathological examination of the resected tumor. (H&E stain) (a) Scanner image (40X) showing signet ring cells (arrow) in centre; (b) High resolution (400X) showing signet ring cells.
Discussion
Bladder exstrophy is a rare disease with an incidence of 2.15 per 100,000 births [1]. With the defect being obvious, almost all patients are treated soon after birth. Studies have found that such patients, even after reconstruction, are prone to malignancies. According to Smeulders et al, risk of neoplasia in treated exstrophy patients is 17.5% [2]. The risk of bladder neoplasia in treated exstrophy patients is 4%, that is, 700 fold higher risk than in normal population [2].
It is even rarer, that, an adult presents with untreated exstrophy of bladder after 50 years of age. Such patients have been reported in developed as well as in developing countries. They probably fail to approach health services owing to social neglect and lack of support. Of these, a very small subset presents with a primary bladder carcinoma at the time of presentation.
After extensive search of literature, we came across less than 90 reported cases of primary bladder adenocarcinoma in untreated exstrophy patients. Of these only two such cases were reported from India [3,4].
In 1955, McIntosh and Worley recognized the risk of carcinoma in untreated exstrophy of bladder [2,5]. Of the 40 cases reviewed by him, two-thirds were men and the average age at diagnosis was 44 years (the youngest was 21 years). A total of 82.5% cases were adenocarcinoma, 12.5% were Squamous Cell Carcinoma (SCC), 5% were of unknown type.
The histology of the bladder mucosa in untreated exstrophy has been described in detail by Smeulders et al in 2001 [2]. The trigone is covered by transitional cell epithelium. The epithelium at the top is characterized by squamous metaplasia merging into normal skin in the middle of the bladder, the epithelium shows glandular metaplasia. Considering the range of epithelia lining the untreated exstrophy bladder, various tumors are possible in such patients. The two most common malignancies are adenocarcinoma (75-85%) and SCC (5%) [5,6]. In contrast, only 0.5-2% of bladder cancers in normal population are adenocarcinoma [2,6].
Histological subtypes of adenocarcinoma of bladder can be nonspecific, enteric, mucinous, signet-ring cell, or mixed. In our case, the final histopathology turned out to be signet-ring cell type. Only one other case of signet-ring cell adenocarcinoma in untreated exstrophy patient has been reported in literature, suggesting rarity of this variant [7].
McIntosh and Worley considered that exposure of the exstrophied bladder to the environment and constant infection resulted in glandular metaplasia [5], possibly to produce protective mucus, and that this was the site of malignant change. It is also suggested that adenocarcinoma in exstrophic bladder originates from the colonic epithelium covering the mucosa of the organ. However, accurate prospective studies are lacking to confirm these theories. Furthermore, rarity of such cases makes a prospective study quite difficult. Smeulders concluded that chronic irritation and infection leading to metaplastic transformation of urothelium resulting in malignant changes is the most likely possibility [2].
Treatment of such a case remains primarily surgical. Systemic chemotherapy has been found to be ineffective for non-urothelial carcinoma cases like adenocarcinoma and SCC [8,9]. After ruling out other sites of primary adenocarcinoma, radical cystectomy with wide local excision of skin margin followed by ileal conduit is ideal for such cases.
Lower abdominal defect repair after exstrophic bladder excision is a challenge because of the large defect seen usually and widely separated rectus in such patients because of pubic diverication. So the options to reconstruct the defect were rectus abdominis rotation flap, fascio-cutaneous M-plasty [10], Cardiff repair with onlay mesh repair [11,12].
We preferred rectus abdominis rotation flap as it was based on inferior epigastric artery and locally raised and mesh was not required which has its own postoperative complications.
At one year follow-up of patient, there was no recurrence or metastasis. After fourteen months of surgery, patient died secondary to myocardial infarction.
Conclusion
An adult seeking treatment for untreated exstrophy and primary adenocarcinoma of bladder is an extremely rare yet distinct possibility. We recommend radical cystectomy with ileal conduit as primary treatment of such patients after ruling out metastasis and other primary sites. The significant lower abdominal defect left behind can be closed with rectus abdominis rotation flap.
[1]. Nelson CP, Dunn RL, Wei JT, Contemporary epidemiology of bladder exstrophy in the United StatesJ Urol 2005 May 173(5):1728-31. [Google Scholar]
[2]. Smeulders N, Woodhouse C R, Neoplasia in adult exstrophy patientsBJU International 2001 87:623-28. [Google Scholar]
[3]. Shwetha S, Ghalige HS, Goyal L, Jain P, Fakhruddin Oncologic concerns in an exstrophied urinary bladder – An Indian ScenarioJ Clin Diagn Res 2015 9(9):XD04-05. [Google Scholar]
[4]. Bhadoo D, Bajpai M, Panda SS, Vijay MK, Sharma N, Adenocarcinoma of exstrophy bladder in a child with gender dysphoria: A rare entity with possible managementJournal of Progress in Paediatric Urology 2013 16(1) [Google Scholar]
[5]. McIntosh JF, Worley G, Adenocarcinoma arising in exstrophy of the bladder : report of two cases and review of literatureJ Urol 1955 73:820-29. [Google Scholar]
[6]. Vozelgang NJ, Comprehensive Textbook of Genitourinary Oncology:379 [Google Scholar]
[7]. Di Lauro G, Iacono F, Ruffo A, Romis L, Mordente S, Pane U, Presenting a case of a mucinous adenocarcinoma of an exstrophic bladder in an adult patient and a review of literatureBMC Surgery 2013 13(Suppl 2):S36 [Google Scholar]
[8]. Montie JE, Eisenberger MA, NCCN Clinical Practice Guidelines in OncologyBladder Cancer (including Upper tract tumors and urothelial carcinoma of Prostate) 2008 V.2Available online at http://www.nccn.org/professionals/physician_gls/PDF/bladder.pdf [Google Scholar]
[9]. Seifker-Ratke AO, Dinney CPN, Czerniak B, Milikan RE, Bladder Cancer: In MD Anderson Manual of Medical Oncology. Kantarjian HM, Wolf RA, Koler CA editors 2007 1st EditionNew DelhiTata McGraw Hill Publishers:739-56. [Google Scholar]
[10]. Shoukry AI, Shoukry I, Management of bladder exstrophy in adulthood: report of 5 casesJ Pediatr Urol 2013 9(5):575-78. [Google Scholar]
[11]. Bansal P, Gupta A, Mongha R, Kundu AK, Squamous cell carcinoma in exstrophic unreconstructed urinary bladder in an adultSaudi J Kidney Dis Transpl 2012 23(1):122-24. [Google Scholar]
[12]. Shukla VK, Mongha R, Gupta N, Chauhan VS, Incisional hernia-comparison of mesh repair with Cardiff repair: An university hospital experienceHernia 2005 9(3):238-41. [Google Scholar]