Introduction
Haemovigilance, the term derived by amalgamation of Greek word ‘haema’ means blood and a Latin word, ‘vigil’ means watchful. The concept of haemovigilance first came into existence in France in 1990, almost with same ideas and vision of pharmacovigilance [1]. As per International Haemovigilance Network (IHN) and International Society of Blood Transfusion (ISBT), haemovigilance is described as an assembly of surveillance strategies covering the complete transfusion sequence from the collection of blood and its components to the follow up of its recipients, designed to collect and appraise information on unexpected or undesirable reactions resulting from the therapeutic use of blood products, and to avoid their occurrence and recurrence [1,2]. So, haemovigilance is an organised system that incorporates monitoring, identification, reporting, investigating and analysis of adverse episode near-misses and reactions pertinent to transfusion and manufacturing blood products. A near-miss episode represents a flaw or deviation from standard procedures or practices that is detected before the outset of the transfusion and that could have led to an improper transfusion or to a reaction in a recipient [3]. This approach was developed by the French Blood Agency in 1994 with the establishment of monitoring schemes by Blood Transfusion Committees and setting up a national haemovigilance system [4]. This system is also an essential component of quality control in a blood system, prompting remedial and preventive measures, and for the perpetual advancement of the quality and safety of blood products and the transfusion process. Nowadays haemovigilance systems have been enforced all over the globe in most developed countries, to monitor the adverse events and episodes related to blood donations and transfusions. It is now well-recognized as an integral part of quality management system in a blood program in many countries [5].
Although, over the last few decades, several countries have established haemovigilance systems to revamp the safety of transfusion process, there are important variations in haemovigilance systems among the countries in terms of the organizational set up, reporting requisites, coverage of whole or part of the transfusion sequence, and the level of development of the health care system in totality. Due to the absence of national mechanisms to report and manage data pertinent to the transfusion process, and also due to meagre traceability between the donors and patients, these systems may not be established at national level, but functioning at institutional or regional level [6]. Approximately, 107 million units of blood donations are collected globally every year. Nearly 50% of these blood donations are collected in high-income countries. Blood donation rate in developed countries is 39.2 donations per 1000 population; 12.6 donations in developing and 4.0 donations in underdeveloped countries [7].
History of Blood Transfusion and need for Haemovigilance
In the 17th century, the first blood transfusions were attempted to transfuse humans with animal blood for all types of ailments. In the 18th century, the French King Louis XIV legally banned the transfusion of animal blood to human because it was considered unsafe and deadly [8]. In the 19th century, Henri Leacock and James Blundell first explored inter-human transfusion as a life saving remedy for severe blood loss [9]. Blundel however proposed to use this remedy only as ultimum refugium; an ultimatum and when all other alternative treatment modalities failed, because the procedure was also risky [9]. However, in the 20th century, due to discovery of ABO blood groups and evolution of cross-matching technique and anti-coagulation used in the blood, blood transfusion became less risky and an accepted treatment modality [10]. Still it was realized that transfusion was certainly not without risk, data were lacking about the actual risk of blood transfusion. At the end of the 1980s, the transmission of infections by blood prompted the need for a greater awareness on the safety of blood and pioneer work on haemovigilance started in France in 1991 with the setup of monitoring systems by blood transfusion committees, resulting in a national haemovigilance network in 1994 [11,12].
Global scenario of haemovigilance: International collaboration and development of IHN.
With the pioneering work on haemovigilance started in France in 1994, later in 1995, the European Council published a resolution with an objective to improve public confidence in safe blood supply. Thus, the haemovigilance system became governed by legal authorities [13,14]. In 1997, the initiative was taken to establish the European Haemovigilance Network (EHN) with the aim to increase the safety of clinical blood transfusion service in Europe. The network started with 5 members from Europe and finally grew to 28 members, including 7 from outside Europe. The scope of EHN gradually broadened which resulted in the development of IHN [2,5]. The aim of IHN is to establish and continue a joint network relating to the safety of blood and blood components and of haemovigilance in blood transfusion and transfusion medicine globally. The intentions are interchange of authentic and credible information between the members, rapid alert / early warning between the members, joint activities between the members of the network, and educational activities in relation to haemovigilance [1]. The activities of the IHN have provided an important contribution to the high quality of haemovigilance in Europe through digital information exchange, meetings and seminars. On standardization, the IHN and the ISBT working party for haemovigilance have contributed two important standardizations: the definitions of adverse reactions and adverse events in patients, and the incitement and management of donor vigilance including the uniformity of definitions of complications and adverse events in donors. Between 2003 and 2005, the European Commission published European Blood Directives that give mandatory rules for collection, testing, processing, storage and distribution of human blood and blood components [13]. The directives dealing with haemovigilance require: 1) Full traceability of blood products from donor to recipient; 2) Notification of a) Serious Adverse Events (SAE) that may have an influence on the recipient; and b) Serious Adverse Reactions (SAR) that may be attributed to the quality and safety of blood and blood components and thus consolidate the support and services of haemovigilance network in a more stringent manner [13–15].
Haemovigilance program in the developed countries is linked to IHN and have a voluntary reporting requirement [2]. At France, Germany, Switzerland, haemovigilance system is governed by regulators; in Japan, Singapore, South Africa, it is under blood manufacturers; in Netherlands and UK, it is under medical societies; and in Canada it is regulated by public health authorities. Some well-established haemovigilance systems of various countries are United Kingdom {Serious Hazards of Transfusion (SHOT)}, Netherlands {Transfusion Reactions in Patients (TRIP)}, Canada {Transfusion Transmitted Injuries Surveillance System (TTISS)}. These medical bodies have provided vision into various measures in pharmacovigilance system and help in improving blood safety. Well established haemovigilance systems are inadequate or lacking among Asian countries and there is lack of haemovigilance data also [13–16].
As per World Health Organization (WHO) Global Database on Blood Safety Report 2004-2005, a national Haemovigilance system is present in 42 (40%) of the 105 reporting countries, with 24 countries (23%) being in the process of development of such a system [17]. There is paucity of data on the use of donated blood. Analysis of data from 57 countries shows that only 14% of developing and transitional countries have >50% hospitals with transfusion committees. Even only 58% of developed countries have >50% hospitals with transfusion committees functioning. These data prove that the "blood usage" and "monitoring of transfusion practice" are health concerns of the whole world and there are major gaps in systems for monitoring transfusion policies practices [18].
Scope of Haemovigilance, its Essentiality and Terminology
Purview of different haemovigilance systems differ due to differences in range of reporting. Ideally, the haemovigilance system should cover all measures and techniques throughout the whole transfusion sequence, from blood donation, processing, and transfusion to patients for the monitoring, reporting, and investigation of adverse events and reactions and near misses pertinent to blood transfusion [1]. It should be well integrated between the blood transfusion service, hospital staff and transfusion laboratories, transfusion committees, regulatory authorities, and national health agencies. An adverse event that results in morbidity or mortality of a recipient is called an adverse reaction and when it affects a donor it is called complication.
Haemovigilance for Recipients
An internationally accepted scale is used to grade the ‘severity’ of an adverse reaction in recipient. The likelihood for adverse reaction or imputability can be attributed to the blood component transfused and it is also important to determine whether blood component has been involved or not. Criteria for ‘severity’ and ‘imputability’ of transfusion reactions have been laid down by the ISBT [19]. Internationally accepted definitions for adverse reactions in recipients have been developed by IHN-ISBT working committee group in order to be able to share information and compare data [20].
Haemovigilance for Donors
Blood donor haemovigilance is also equally important as far as adverse reaction or event during whole blood or component donation is concerned. Adverse reaction in donor is called complication as the aetiology is different from those in the recipient. These adverse reactions may be due to donation, selection, and management of donors, which may directly harm the donor or impact the quality of the product, which ultimately influence the recipient. A classification and a set of definitions of complications have been proposed by a joint working group from the ISBT and EHN [19]. They are subdivided in local reactions related to needle insertion (vessel injuries, nerve injuries, other), general reactions (vasovagal immediate and delayed type) and other important complications. The severity and imputability of donor complications are graded according to another but comparable scale as used for adverse reactions in recipients. This scale is also internationally accepted [21].
Transfusion Practices and Haemovigilance in Developing Countries
Although the substantial level of awareness on transfusion transmitted diseases has already attracted considerable attention from transfusion medicine professionals, no significant advances have been made to minimize preventable transfusion errors in developing countries. There is a lack of awareness and proper training about the management of transfusion related adverse reactions among health workers and this leads to under-reporting of transfusion errors. Many African countries do not have an effective haemovigilance system and very few data regarding transfusion incidents in Africa are available [22]. In India, there is a lack of standardized and effective haemovigilance system as the reporting of adverse transfusion event is not mandatory [23]. Furthermore, there is under-reporting by the medical staff and thus many adverse reactions do not come into attention. However, with gradual increase in awareness over the last few years on haemovigilance and blood safety, many institutes and centres across India have recently published significant data on adverse transfusion events [23–27]. Thus, considering the gravity of the problem, a national haemovigilance program as an integral part of the PvPI at a national level was launched on December 10, 2012 [28].
WHO has taken some initiatives in order to support and consolidate the haemovigilance program in resource poor countries. The goal of these initiatives is to strengthen and expand national systems for data collection and management, risk assessment, surveillance and vigilance for policy decisions and programme planning for safe blood transfusion. WHO has developed norms, standards, recommendations, guidelines, materials tools and training materials which will be useful for countries in developing haemovigilance systems. This will help in assessment, monitoring and evaluation of national blood programme. WHO has also established a mechanism of collecting and reporting data of blood transfusion services from 194 WHO Member States (annually) based on 20 key quantitative blood safety indicators and (triennially) using a comprehensive data collection tool. In 2007, WHO organized a "Global Consultation on Universal Access to Safe Blood Transfusion". The international experts and participants of this consultation gave recommendations to WHO on developing quality systems throughout the Blood transfusion chain [29]. WHO has recently initiated the establishment of a Global Haemovigilance Network, building on the existing efforts and in collaboration with IHN, ISBT and Federal Govt. of Canada (Public Health agency of Canada and Health Canada). This initiative will focus on the needs of developing countries in establishing haemovigilance systems and will also explore the possibilities of international data and information sharing. In November 2012, WHO organized a global consultation jointly collaborated with IHN and ISBT in Dubai, United Arab Emirates and laid down recommendations on recent development on haemovigilance [30].
National Haemovigilance Program of India
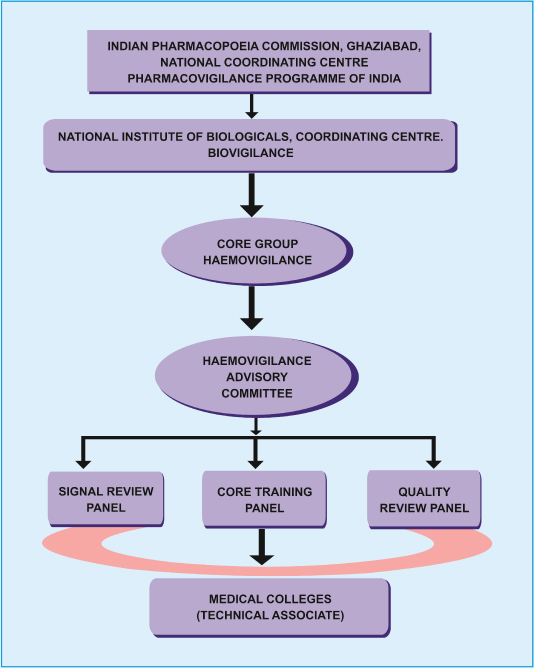
Indian Pharmacopoeia Commission in collaboration with National Institute of Biologicals, Noida, Uttar Pradesh has launched a HvPI on 10th December 2012 across the country under its PvPI, under Ministry of Health and Family welfare, Government of India. This program has been Implemented under the broad ambit of PvPI with dedicated budgetary provision Rs 29.36 crore during 12th Five Year Plan (2012-2017) and divided into three phases (initiation phase for financial year 2012-13, expansion and consolidation phase for financial year 2013-15 and expansion and maintenance phase for financial year 2015-17) for establishment of haemovigilance program [31]. This is an independent program primarily restricted to voluntary reporting of serious adverse reaction in recipients. Fundamental aim of this program is to trail adverse reactions and episodes related to blood transfusion and blood product administration and to help determine the tendency, recommend best practices/policies and interventions required to improve patient care and safety. A software -“Haemo-Vigil” has been developed to collect and analyse the data related to haemovigilance all over the country. For HvPI to collect and analyse data related to biologicals and haemovigilance, National Institute of Biologicals is acting as the co-ordinating centre, The ultimate goal of this HvPI is to be a part of the IHN which presently has 28 countries as its member and provides a global forum for sharing best practices and benchmarking of haemovigilance data. An organogram regarding the functions of haemovigilance program has been delineated by the joint recommendation of Indian Pharmacopoeia Commission and National Institute of Biologicals (IPC-NIB) [Table/Fig-1].
Haemovigilance organogram [31].
Objective of Reporting Adverse Reactions and in Transfusion in National Haemovigilance Program
The core group of advisory committee has already formulated a guidance document on transfusion reaction reporting with the main objective of obtaining information which can be used to improve the transfusion safety [31].
• A national reporting system which can be regarded as a measure of advance public policy relating to patient safety.
• Reporting can determine hazards and risks, and provide information as to where the system is at fault.
• This can help to reduce the probability of injury to future patients.
• Prompt reporting facilitates effective risk management.
Documenting and Reporting of Serious Adverse Reactions/Events in Blood & Blood products Transfusion
There are several types of transfusion reactions [1,32], which can be subdivided in different ways according to the time of occurrence, pathogenesis and / or symptomatology. According to the time of occurrence, it is subdivided as acute (< 24 hours after transfusion) and delayed (> 24 hours after transfusion) reactions. As per their pathogenesis, adverse reactions can be further divided as infectious and non-infectious adverse reactions. Major non-infectious acute reactions include Acute Haemolytic Transfusion Reactions (AHTR), Febrile Non-Haemolytic Transfusion Reactions (FNHTR), allergic reactions including anaphylactic reactions, Transfusion Associated Acute Lung Injury (TRALI), Transfusion Associated Circulatory Overload (TACO), hypotensive reactions and hyperkalemia. Non-infectious delayed transfusion reactions are Delayed Haemolytic Transfusion Reactions (DHTR), Delayed Serological Transfusion Reactions (DSTR), Post-Transfusion Purpura (PTP), Transfusion-Associated Graft Versus Host Disease (TAGVHD) and haemosiderosis.
The major acute infectious adverse reactions [1] are due to bacterial contamination of the blood component, and delayed infectious reactions may be due to viral (e.g., hepatitis B / C, HIV) or parasitic (e.g., malaria) transmission. It is therefore important to know the frequency at which different reactions can occur so that the interventions can be prioritized in order to improve the transfusion safety. Out of all transfusion reactions, FNHTR (1000-3000 per 100,000 units transfused) and allergic reactions (112.2 per 100000 units transfused) are most common form of reactions [32]. Regarding documenting and reporting of transfusion reactions, a reporting format, Transfusion Reaction Reporting Form (TRRF) [33] has been prepared by HvPI, which mentions all information regarding the patient, transfusion reaction details, blood component or blood product details, list of relevant and necessary investigations needed to be done, nature of adverse reaction and imputability assessment. This TRRF is freely available in the website of HvPI [33]. All the medical colleges in India have been encouraged to enrol under HvPI and upload the transfusion related adverse events through the haemovigil software after filling up the transfusion reaction reporting form. The data collected through this software will be collated and analysed to determine the trends and propose appropriate practices and interventions mandatory to improve patient care and safety [28].
HvPI has received a very good response as most of the medical colleges and Institutes have already enrolled and started providing data on adverse reactions. However, less attention has been paid to donor haemovigilance. Hence, a National Blood Donor Vigilance Programme (NBDVP) was started on 14th June, 2015 on the World Blood Donor Day under the scope of HvPI [34]. The main objectives of the NBDVP are to improve donor safety and satisfaction through monitoring and analysing the risk factors, implementing preventing measures with an ultimate goal of reducing the frequency of adverse donor reactions and increasing donation frequency [34]. A one page Adverse Donor Reaction Reporting Form (ADRRF) has been developed to collect information about adverse events or complications related to blood donation. This form has been prepared in line with the complications related to blood donation as formulated by ISBT working group on donor vigilance [35]. ADRRF is also freely available in the website of HvPI [36] and all medical colleges are encouraged to get enrolled under the HvPI so that any adverse reactions associated with blood donation collected through ADRRF will be collated and analysed through the haemovigil software. ADRRF mentions all details which include donor information, details of blood collected, type of complications, outcome of adverse reaction and imputability [36]. This practice will help in identifying the trends and recommend best practices and interventions required to improve donor safety as well [34].
However, documenting and reporting Transfusion Reactions in blood transfusion service involve many aspects and interrelationships among various departments:
1. Responsibilities of medical and nursing staff of the ADR monitoring centres.
2. Responsibilities of the transfusion service department of the ADR monitoring centres.
3. Responsibilities of the hospital transfusion committee of the ADR monitoring centres.
4. Responsibilities of the head, department of transfusion of the ADR monitoring centres.
5. Responsibilities of the technical associate IPC –PvPI posted in the ADR monitoring centres.
6. Responsibilities of haemovigilance centre, National Institute of Biologicals (NIB).
7. Responsibilities of PvPI, National Co-coordinating Centre (NCC), Indian Pharmacopoeia Commission (IPC) [31].
8. Responsibilities of Central Drugs Standard and Control Organization (CDSCO), New Delhi.
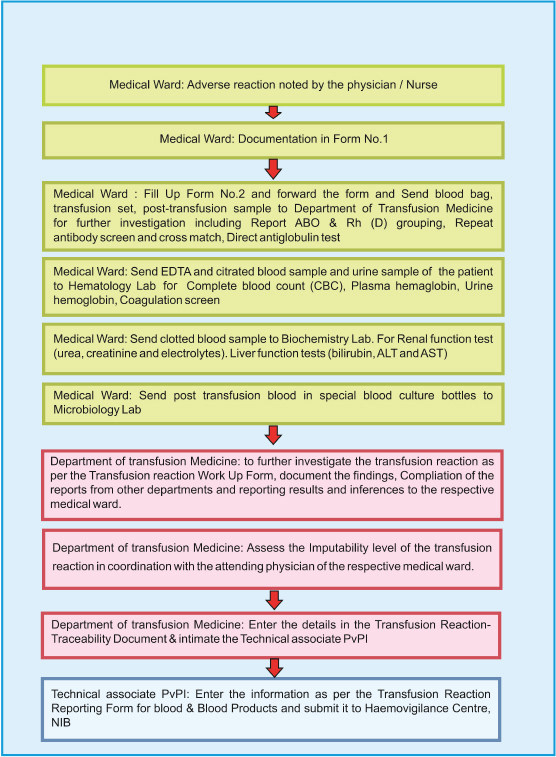
A flow chart format for reporting serious adverse reaction on blood transfusion along with the roles and responsibilities of medical and nursing staff in the ward as well as the staff in the department of transfusion medicine have been described in the guidance document of IPC-NIB [Table/Fig-2]. The department of transfusion medicine in any hospital plays a major role in evaluating the adverse reactions which includes the assessment of imputability of adverse reactions in coordination with the attending physician.
Flow chart for reporting serious adverse reactions in blood transfusion [31].
Roles and Responsibilities of HvPI and CDSCO Units
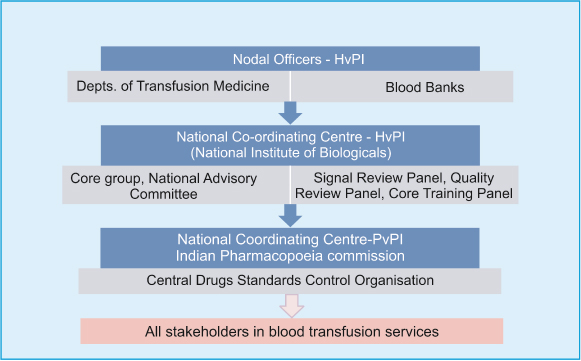
The data on adverse transfusion reactions and events are entered into the “haemovigil” software from the transfusion medicine department/blood bank/hospitals/medical colleges and transmitted to HvPI-NCC, NIB. HvPI-NCC reviews completeness of data quality, prepare SOPS, guidance documents and communicate recommendations of to IPC. IPC finally forwards recommendations of haemovigilance advisory committee to Drug Controller General of India (DCGI)-CDSCO body. It is the DCGI-CDSCO who formulates blood and blood product transfusion safety related regulatory decisions and communicate to stakeholders [Table/Fig-3].
Organisational structure for flow of information [31].
HvPI unit is also involved in preparation of training materials in the form of publications of haemovigilance newsletter, Information, Education and Communication (IEC) literatures and conducting academic CMEs, awareness program on haemovigilance throughout the year in India. As per latest newsletter on haemovigilance, 206 centres including hospitals, medical colleges and blood transfusion centres have been enrolled under this program. A total of 2296 Transfusion Reactions have been received by NCC [37], Haemovigilance program in India has now become member of International Haemovigilance Network (IHN). Therefore, the major recommendations for better haemovigilance program must incorporate better national blood quality and safety initiatives, reducing or minimizing human errors, recruiting more trained personnel, generate data standard and improved reporting capacity [38].
Conclusion
Haemovigilance program is an integral part of pharmacovigilance program of India which scrutinizes, expedite remedial and preventive actions to be taken to minimize or reduce the potential risks related to safety and quality in blood processing and transfusion for donors, patients and medical staff. Thus it incorporates required modifications in the relevant policies, improve standards, assists in the formulation of guidelines, and reinforce the safety and quality of the whole chain from donation to transfusion. In resource constrained countries, a stepwise implementation of the policies would be required in order to establish guidelines and develop haemovigilance systems in a substantial manner. Haemovigilance will also have a major impact on optimal blood usage. It is expected that existing haemovigilance systems in hospitals will contribute in the near future also to the surveillance of optimal blood use.
[1]. De Vries RR, Faber JC, Strengers PF, Haemovigilance: An effective tool for improving transfusion practiceVox Sang 2011 100:60-67. [Google Scholar]
[2]. Proposed standard definitions for surveillance of non-infectious adverse transfusion reactions [Internet]. International haemovigilance network; 2011. Available from: http://www.isbtweb.org/fileadmin/user_upload/WP_on_Haemovigilance/ISBT_definitions_final_2011_.pdf. [Accessed on September 21,2015] [Google Scholar]
[3]. Faber JC, Haemovigilance: Definition and overview of current haemovigilance systemsTransfusion Alternatives in Transfusion Medicine 2003 5(1):237-45. [Google Scholar]
[4]. De Vries RR, Haemovigilance: Recent achievements and developments in the near futureISBT Sci Ser 2009 4:60-62. [Google Scholar]
[5]. Engelfreit CP, Reesink HW, International Forum: HaemovigilanceVox Sang 2006 90:207-41. [Google Scholar]
[6]. Faber JC, Worldwide overview of existing haemovigilance systemsTransfus Apheresis Sci 2004 31:99-110. [Google Scholar]
[7]. World Health Organization; Global database on blood safety. Report 2004-2005. Available from: http://www.who.int/bloodsafety/global_database/GDBSReport2004-2005.pdf [Accessed on September 23, 2015] [Google Scholar]
[8]. Hoff HE, Guillemin R, The first experiments on transfusion in FranceJ Hist Med 1963 18:103-24. [Google Scholar]
[9]. Schmidt PJ, Leacock AG, Forgotten transfusion history: John Leacock of BarbadosBMJ 2002 325(7378):1485-87. [Google Scholar]
[10]. Bhattacharya P, Marwaha N, Dhawan HK, Roy P, Sharma RR, Transfusion-related adverse events at the tertiary care center in North India: An institutional haemovigilance effortAsian Journal of Transfusion Science 2011 5(2):164-70. [Google Scholar]
[11]. Noel L, Debeir J, Cosson A, The French haemovigilance systemVox Sang 1998 74(Suppl 2):441-45. [Google Scholar]
[12]. Andreu G, Morel P, Forestier F, Debeir J, Rebibo D, Janvier G, Haemovigilance network in France: organization and analysis of immediate transfusion incident reports from 1994 to 1998Transfusion 2002 42(10):1356-64. [Google Scholar]
[13]. Faber JC, The European Blood Directive: A new era of blood regulation has begunTransfus Med 2004 14:257-73. [Google Scholar]
[14]. Watson R, EU tightens rules on blood safetyBMJ 2005 331:800 [Google Scholar]
[15]. Quality and safety standards for human blood and blood components. Summaries of EU legislation. Available from: http://europa.eu/legislation_summaries/public_health/threats_to_health/c11565_en.htm [Accessed on September 25, 2015] [Google Scholar]
[16]. Public Health Service (PHS) Biovigilance working group, Department of Health and Human Services Washington, DC20201. Biovigilance in the United States: Efforts to bridge a critical gap in patient safety and donor health. 2009;16 [Google Scholar]
[17]. World Health Organization: Global Database on Blood Safety. Report 2004-2005 [Google Scholar]
[18]. Dhingra N. Haemovigilance in developing countries. Available from: http://www.bloodtransfusion.it/articoli/47/en/Doi%200010.pdf [Accessed on September 25, 2015] [Google Scholar]
[19]. Stainsby D, Faber JC, Jorgensen J, Overview of haemovigilance. In: Simon TL, Solheim BG, Straus RG, Snyder EL, Stowell CP, Petrides M, editorsRossi’s Principles of Transfusion Medicine 2009 4th edWest SussexBlackwell Publishing:694 [Google Scholar]
[20]. Proposed Standard Definitions for surveillance of non infectious adverse transfusion reactions JULY 2011 incorporating correction to TRALI definition (as adopted June 2013). ISBT working party on haemovigilance Available from: http://www.isbtweb.org/fileadmin/user_upload/Proposed_definitions_2011_surveillance_non_infectious_adverse_reactions_haemovigilance_incl_TRALI_correction_2013.pdf [Google Scholar]
[21]. Jorgensen J, Sorense BS, Donor vigilanceISBT Sci Ser 2008 3(1):48-53. [Google Scholar]
[22]. Tayou Tagny C, Mbanya D, Tapko JB, Lefrère JJ, Blood safety in Sub-Saharan Africa: a multi-factorial problemTransfusion 2008 48:1256-61. [Google Scholar]
[23]. Vasudev R, Sawhney V, Dogra M, Raina TR, Transfusion-related adverse reactions: From institutional haemovigilance effort to National Haemovigilance programAsian Journal of Transfusion Science 2016 10(1):31-36. [Google Scholar]
[24]. Negi G, Gaur DS, Kaur R, Blood transfusion safety: A study of adverse reactions at the blood bank of a tertiary care centerAdvanced Biomedical Research 2015 4:237 [Google Scholar]
[25]. Sharma DK, Datta S, Gupta A, Study of acute transfusion reactions in a teaching hospital of Sikkim: A haemovigilance initiativeIndian Journal of Pharmacology 2015 47(4):370-74. [Google Scholar]
[26]. Kumar P, Thapliyal R, Coshic P, Chatterjee K, Retrospective evaluation of adverse transfusion reactions following blood product transfusion from a tertiary care hospital: A preliminary step towards haemovigilanceAsian Journal of Transfusion Science 2013 7(2):109-15. [Google Scholar]
[27]. Philip J, Pawar A, Chatterjee T, Mallhi RS, Biswas AK, Dimri U, Non Infectious Complications Related to Blood Transfusion: An 11 year Retrospective Analysis in a Tertiary Care HospitalIndian J Hematol Blood Transfus 2016 32(3):292-98. [Google Scholar]
[28]. Bisht A, Singh S, Marwaha N, Haemovigilance Program-IndiaAsian J Transfus Sci 2013 7:73-74. [Google Scholar]
[29]. Universal Access to Safe Blood Transfusion. World Health Organization, Department of Essential Health Technologies, Blood Transfusion Safety Unit, 2008. Available from: http://www.who.int/bloodsafety/publications/UniversalAccesstoSafeBT.pdf [Accessed on September 25, 2015] [Google Scholar]
[30]. World Health Organization: Blood Transfusion Safety. Available from: http://www.who.int/bloodsafety/haemovigilance/RecommendationsGlobal ConsultationHaemovigilance.pdf [Accessed on September 21,2015] [Google Scholar]
[31]. IPC-NIB guidance document for reporting serious adverse reactions in blood transfusion service. National Institute of Biologicals & Indian Pharmacopoeia Commission Collaboration. Available from http://nib.gov.in/haemovigilance.html [Accessed on September 21,2015] [Google Scholar]
[32]. Delaney M, Wendel S, Bercovitz RS, Cid J, Cohn C, Dunbar NM, Apelseth TO, Popovsky M, Stanworth SJ, Tinmouth A, Van De Watering L, Waters JH, Yazer M, Ziman A, Biomedical Excellence for Safer Transfusion (BEST) Collaborative. Transfusion reactions: prevention, diagnosis, and treatmentLancet 2016 :piiS0140-6736(15)01313-16 [Google Scholar]
[33]. Haemovigilance Programme; National Institute of Biologicals. Available from: http://nib.gov.in/Haemovigilance/TRRF_Form.pdf [Accessed on August 13, 2016] [Google Scholar]
[34]. Bisht A, Singh S, Marwaha N, National blood donor vigilance programme: IndiaAsian J Transfus Sci 2016 10:1-2. [Google Scholar]
[35]. Standard for Surveillance of Complications Related to Blood Donation, Working Group on Donor Vigilance of the International Society of Blood Transfusion Working Party on Haemovigilance in collaboration with The International Haemovigilance Network The AABB Donor Haemovigilance Working Group, December 11, 2014, available from: https://www.aabb.org/research/haemovigilance/Documents/Donor-Standard-Definitions.pdf. [Accessed on August 12, 2016] [Google Scholar]
[36]. Haemovigilance Programme; National Institute of Biologicals Available from http://nib.gov.in/Haemovigilance/DARR_Form.pdf [Accessed on August 14, 2016] [Google Scholar]
[37]. Haemovigilance Programme of India News Letter 2015 3(6):2-3. [Google Scholar]
[38]. Sen S, Gupta P, Sinha S, Bhambani P, Haemovigilance and transfusion safety: A ReviewSch J App Med Sci 2014 2(1A):85-90. [Google Scholar]