Unusual Visceral Sarcomas: Report of 2 Cases with Review of Literature
Navneet Jain1, Nadia Shirazi2, Neena Chauhan3, Meenu Gupta4
1 Assistant Professor, Surgical Oncology, Himalayan Institute of Medical Sciences, Jolly Grant, Dehradun, Uttarakhand, India.
2 Associate Professor, Pathology, Himalayan Institute of Medical Sciences, Jolly Grant, Dehradun, Uttarakhand, India.
3 Professor, Pathology, Himalayan Institute of Medical Sciences, Jolly Grant, Dehradun, Uttarakhand, India.
4 Associate Professor, Radiation Oncology, Himalayan Institute of Medical Sciences, Jolly Grant, Dehradun, Uttarakhand, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Nadia Shirazi, B-IX-6, HIHT Campus, Himalayan Institute of Medical Sciences, Jolly Grant, PO. Doiwala, Dehradun-248140, Uttarakhand, India.
E-mail: shirazinadia@gmail.com
Sarcomas account for only 1% of adult solid tumours. Visceral sarcomas except Gastrointestinal Stromal Tumours (GIST) are rare and therefore little is known about the natural history and prognosis of these tumours. They tend to occur in older adults with no sex predilection and are characterized by an aggressive behaviour. Proper evaluation of these tumours is necessary because these are uncommon tumours which often present with advanced disease in an anatomically complex location. Since there are very few published studies on visceral sarcomas, the data is insufficient to suggest prognosis and optimum treatment strategies. We present two cases of such unusual malignancies in spleen and urinary bladder.
High grade, Intra-abdominal, Radiation induced
Case Report
Case 1
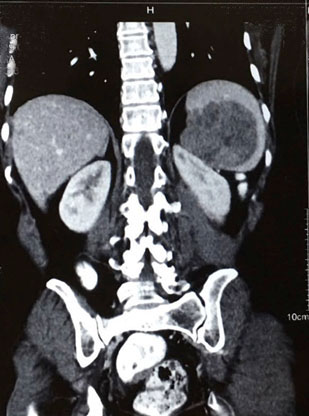
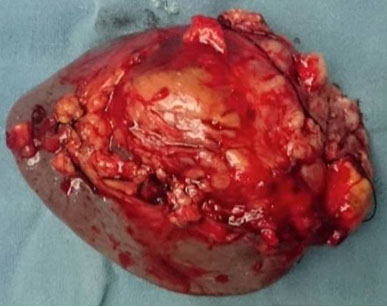
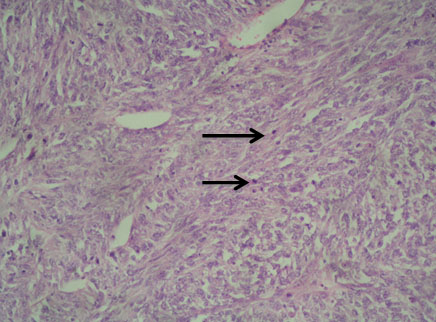
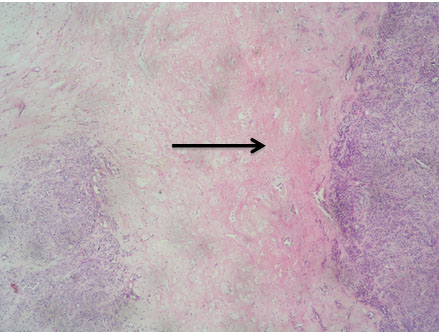
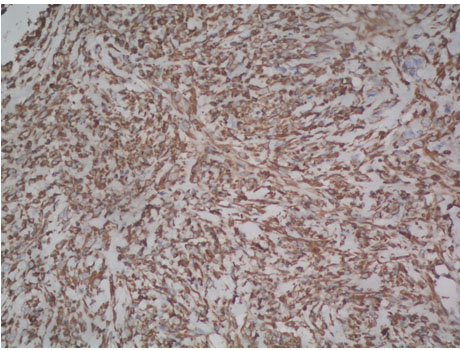
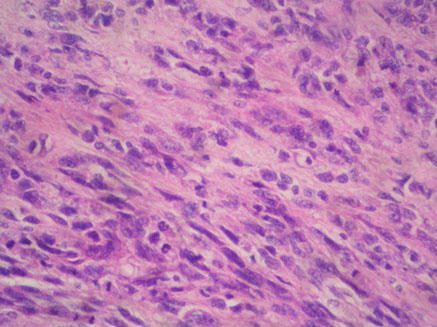
A 54-year-old North Indian female presented with a history of pain in lower abdomen since 1 month and decreased appetite since 15 days. The patient was treated for carcinoma ovary 4 years back for which she had undergone cytoreductive surgery, chaemotherapy as well as radiotherapy. On examination, spleen was palpable 3 cm below the costal margin. Hepatomegaly, lymphadenopathy and ascites were absent. Chest X-ray showed normal lung fields and mediastinum. Magnetic Resonance Imaging (MRI) of abdomen showed a large heterogeneous mass in spleen measuring 7x6 cm [Table/Fig-1]. A differential diagnosis of splenic hematoma or splenic metastasis was considered, for which exploratory laparotomy with splenectomy was performed and sent for histopathological examination. Gross examination of spleen revealed a poorly encapsulated grey white tumour measuring 7x6x5cm. Tumour was replacing almost the entire organ and showed splenic capsular rupture at few places. Tumour had a variegated appearance with a fish flesh consistency and showed areas of haemorrhage and necrosis [Table/Fig-2]. Haemorrhage and necrosis were seen at few places. Microscopic examination showed a cellular high grade spindle cell sarcoma with brisk mitotic activity 10-12/10 HPF (High Power Field) and multiple areas of tumour necrosis [Table/Fig-3,4]. Tumour was infiltrating the hilar vessels microscopically however regional lymph nodes were free of tumour. On Immunohistochemistry tumour cells were Vimentin positive and negative for Cytokeratin (CK), Smooth Muscle Actin (SMA), Desmin and S-100 [Table/Fig-5]. A diagnosis of fibrosarcoma was made. The recovery of the patient was uneventful and she is presently follow-up.
MRI pelvis showing mass in spleen.
Gross photograph of splenectomy specimen showing an infiltrative tumour with areas of haemorrhage;
Photomicrograph of celluar high grade sarcoma in spleen with brisk mitotic activity (Arrow) (H&E, 10X);
Photomicrograph of spindle cell sarcoma with large areas of necrosis (Arrow) (H&E, 4X).
Photomicrograph of tumour cells exhibiting cytoplasmic positivity for Vimentin (Peroxidase-antiperoxidase, 20X);
Case 2
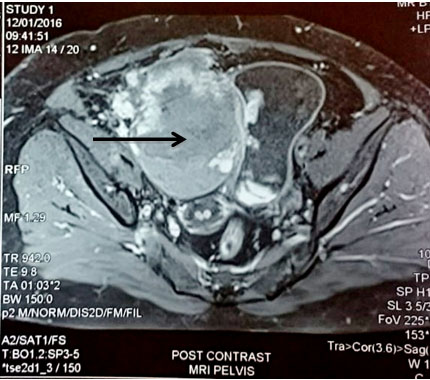
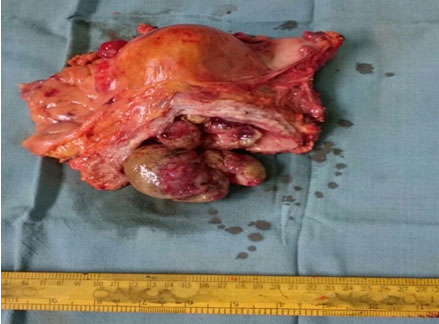
A 62-year-old North Indian female presented with history of haematuria, dysuria and bilateral flank pain since 6 months. For the past 20 days she observed a lump in lower abdomen. There was no history of haematuria or calculi in bladder or kidneys. Her general and systemic examinations were unremarkable. Chest X-ray revealed clear lung fields. MRI pelvis showed multiple urinary bladder growths, largest measuring 10x10x8 cm [Table/Fig-6]. Cystoscopy revealed multiple broad based, polypoidal growths occupying almost the whole of the bladder. Transurethral Resection of Bladder Tumour (TURBT) was done and sent for histopathology, which suggested a malignant mesenchymal tumour following which radical cystectomy was performed. Grossly, specimen measured 18x18x9.5 cm. The entire bladder was replaced by macroscopically a deeply infiltrating lobular tumour measuring 13x10x8 cm. Tumour was infiltrating the walls of bladder and reaching till the perivesical fat [Table/Fig-7]. Microscopically a cellular, high grade spindle cell tumour was seen forming fascicles. Mitotic figures were 15-20/10 HPF. Large areas of tumour necrosis were also seen [Table/Fig-8]. A differential diagnosis of sarcoma versus sarcomatoid variant of urothelial carcinoma was made. Immunohistochemistry showed Vimentin and SMA positivity thus confirming a diagnosis of high grade leiomyosarcoma [Table/Fig-9].
MRI pelvis showing a large mass in the urinary bladder;
Gross specimen of Urinary Bladder showing a polypoidal mass coming out of the urethral orifice and infiltrating the walls of bladder.
Photomicrograph of high grade spindle cell sarcoma in urinary bladder (H&E, 40X).
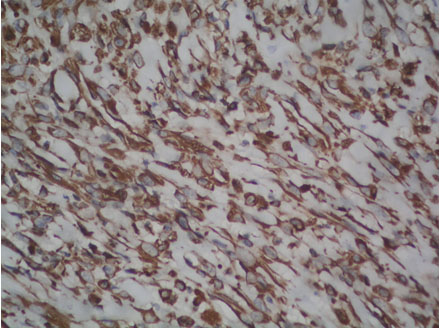
Photomicrograph of tumour cells showing cytoplasmic positivity for Smooth Muscle Actin (Peroxidase anti-peroxidase, 40X).
Discussion
Soft tissue sarcomas of the abdomen and pelvis include a wide variety of both low and high grade tumours. These are subclassified on the basis of their sites of origin i.e., retroperitoneal, intra-abdominal, gynaecological and abdominal wall sarcomas. Non-urothelial malignancies of bladder are rare and account for <5% of all bladder tumours [1].
Visceral sarcomas or intra abdominal sarcomas tend to involve any visceral organ in the abdominal cavity with a predilection for gastrointestinal tract. Histopathologically, Gastrointestinal Stromal Tumour (GIST) and leiomyosarcoma are more frequent subtypes of visceral sarcoma. Visceral sarcomas are rare. Their etiology is unknown and clinical presentation may vary from case to case. Diagnosis can be suspected on Ultrasonography, CT or MRI however exploratory laparotomy with histopathological examination is necessary for confirmation. However by the time a diagnosis is made, tumour usually metastasizes to liver (most frequently), skin, bone, lung, brain and soft tissues [2]. Radical surgical resection (splenectomy) is the treatment of choice and it is still debatable whether adjuvant therapy in the form of radiotherapy and chemotherapy has any beneficial role in long term survival rate of patients [3].
In the indexed Case 1 since splenic sarcoma occurred after the patient was treated for carcinoma ovary, a possibility of radiation induced sarcoma was considered. This type of sarcoma may be the result of intensive cancer treatment combining surgery with adjuvant or neo-adjuvant chaemotherapy and pre operative or postoperative radiation. The precise genetic mechanism of radiation induced sarcoma is largely unknown however it is attributed to genomic stability induced by ionizing radiation [4]. These tumours can develop anytime after radiotherapy are aggressive and carry a worse prognosis as compared to the usual type of sarcomas [5]. In our case the median latency period between irradiation and diagnosis of splenic sarcoma was 4 years which is almost comparable to the published literature where the latency period is roughly 10 years (average 3 months to 50 years) [6,7].
Non epithelial tumours account for <5% of all bladder malignancies with leiomyosarcomas representing 0.1% of all these malignancies [8]. However, leiomyosarcomas constitute the majority of bladder sarcomas (approximately 20%) followed by rhabdomyosarcoma, carcinosarcoma, angiosarcoma and osteosarcoma. There are no clear cut predisposing factors for development of bladder sarcomas however pelvic radiation and chaemotherapy for other carcinomas are considered to play a major role in their etiology.
Lee et al., studied seven bladder soft-tissue sarcomas, on the basis of 28 years of experience with these tumours at one centre in Korea [9]. Tsujita et al., reported a 68-year-old male with a huge bladder diverticulum that infiltrated the mesentery and abdominal wall with multiple pelvic lymph nodes [10]. This was the 3rd case reported in Japan, of a leiomyosarcoma in the urinary bladder diverticulum. Bakaris et al., reported co-existence of 2 distinct and separate primary tumours of bladder i.e., Urothelial carcinoma (TCC) and leiomyosarcoma [11]. The overall local recurrence of bladder leiomyosarcoma is almost 16%, mostly recurring in the pelvis [12]. Therefore, there should be close monitoring with abdomino-pelvic CT, chest X-ray and cystoscopy, especially in the first year after surgery. This might help in the early detection of tumour recurrence and metastasis [13,14].
Conclusion
Since there is limited data on visceral sarcomas, it is not very clear whether the prognosis for such tumours is worse or not particularly after controlling for gender, stage and grade of tumour. All such tumours require a multimodality approach in their treatment.
[1]. Dahm P, Gschwend JE, Malignant non-urothelial neoplasms of the urinary bladder: a reviewEur Urol 2003 44:672 [Google Scholar]
[2]. Townsend C, Mattox KL, Evers MB, Beauchamp RD, Sabiston Text book of surgeryThe biological basis of modern surgical practice 2004 17PhiladelphiaSaunders ed [Google Scholar]
[3]. Dawson L, Gupta O, Garg K, Malignant fibrous histiocytoma of the spleen: an extremely rare entityJ Cancer Res Ther 2012 8:117-19. [Google Scholar]
[4]. Suzuki K, Yokoyama S, Waseda S, Kodama S, Wantanabe M, Delayed reactivation of p53 in the progeny of cells surviving ionizing radiationCancer Res 2003 63:936-41. [Google Scholar]
[5]. Huvos AG, Woodard HQ, Cahan WG, Higinbotham NL, Stewart FW, Butler A, Post radiation Osteogenic sarcoma of bone and soft tissues. A clinicopathologic study of 66 patientsCancer 1985 55:1244-55. [Google Scholar]
[6]. Taghian A, de Vathaire F, Terrier P, Le M, Auquier A, Mouriesse H, Long-term risk of sarcoma following radiation treatment for breast cancerInt J Radiat Oncol Biol Phys 1991 21:361-67. [Google Scholar]
[7]. Huvos AG, Woodard HQ, Cahan WG, Higinbotham NL, Stewart FW, Butler A, Post radiation osteogenic sarcoma of bone and soft tissues. A clinicopathologic study of 66 patientsCancer 1985 55:1244-55. [Google Scholar]
[8]. Feggetter GY, Sarcoma of the bladderBritish J Surg 1937 25:382-87. [Google Scholar]
[9]. Lee G, Lee SY, Seo S, Jeon S, Lee H, Choi H, Prognostic factors and clinical outcomes of urological soft tissue sarcomasKorean J Urol 2011 52:669-73. [Google Scholar]
[10]. Tsujita Y, Sumitomo M, Tasaki S, Shirotake S, Hashiguchi Y, Asano T, A case of leiomyosarcoma in a huge bladder diverticulumActa Urologica Japonica 2009 55:761-64. [Google Scholar]
[11]. Bakaris S, Resim S, Tasci AI, Demirpolat G, A rare case of synchronous leiomyosarcoma and urothelial cancer of the bladderCan J Urol 2008 15:3920-23. [Google Scholar]
[12]. Hamadalla NY, Rifat UN, Safi KC, Mohammed M, Abu-Farsakh H, Leiomyosarcoma of the urinary bladder: A review and a report of two further casesOncology/Reconstruction Review 2013 11:159-64. [Google Scholar]
[13]. Ricciardi E, Maniglio P, Schimberni M, Moscarini M, A case of high-grade leiomyosarcoma of the bladder with delayed onset and very poor prognosisWorld J Surgical Oncol 2010 8:16-18. [Google Scholar]
[14]. Nishiyama H, Habuchi T, Watanabe J, Clinical outcome of a large scale multi-institutional retrospective study for locally advanced bladder cancer: a survey including 1131 patients treated during 1990-2000 in JapanEur Urol 2004 45:176-81. [Google Scholar]