Safety and Efficacy of Itolizumab in the Treatment of Psoriasis: A Case Series of 20 Patients
Anchala Parthasaradhi1
1 Consultant, Department of Dermatologist, Dr. Anchala’s Skin Institute and Research Centre, Hyderabad, Telangana, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Anchala Parthasaradhi, Consultant Dermatologist, Dr. Anchala’s Skin Institute and Research Centre, Hyderabad- 500033, Telangana, India.
E-mail: p_anchala@yahoo.co.in
Psoriasis is a common, chronic, relapsing/remitting, immune-mediated skin disease that causes itchy skin with silvery scales. It is characterized by thickened red erythematous plaques covered with silvery scales. Biological therapies have been recently introduced for patients with psoriasis in India. The biological therapies contain protein biomolecules which can be employed to target specific immune or genetic mediator of a pathophysiological process. Here, we share our clinical experience of managing 20 patients with moderate to severe psoriasis by itolizumab a humanized IgG1 monoclonal antibody. Eighteen patients achieved Psoriasis Area and Severity Index (PASI) 75 response after receiving 10 infusion of itolizumab (at the completion of treatment). Out of 18 patients 4 patients had achieved PASI 95 response and 10 patients had achieved PASI 90 response. There was no adverse event reported during the treatment period. Itolizumab was found effective and safe in the treatment of moderate to severe psoriasis patients.
Biological, Itolizumab, PASI 75, Skin disease
Case Report
A total of 20 patients with moderate to severe psoriasis were registered at a private Skin Institute and Research Centre, Hyderabad An informed consent was taken from the patients. Patients included were from both sexes (15 males and 05 females), with a history of psoriasis for more than 5 years. Chest X-ray and Mantoux test were carried out in patients prior to their registration in the study with a purpose to exclude latent tuberculosis. Routine blood test and other biochemical parameters were also performed at baseline. Patients included, were those who had been initially treated with drug regimens such as systemic methotrexate and cyclosporine with topical medications but with unsatisfactory treatment outcome. Therefore they were treated with Itolizumab (1.6 mg/kg) every fortnight for first 3 months followed by once monthly for next 3 months (total of 10 infusions). Pregnant and lactating subjects were excluded from the study.
They were assessed for demographic details and vital parameters at baseline and completion of treatment. The severity of skin disease was measured using Psoriasis Area Severity Index (PASI) at baseline, visit 7 and visit 10 for all 20 patients [1]. Physician Global Assessment (PGA) score was also measured at baseline and completion of treatment [1].
According to British Association of Dermatologists Guidelines, a PASI score below 3 was defined as “mild,” between 3 and 10 was defined as “moderate,” and above 10 was defined as “severe” disease [2]. The demographic details of the patients are presented in [Table/Fig-1].
Demographic details and PASI score of psoriasis patients.
| PatientNo. | Gender(M/F) | Bodyweight(Kg) | PASIScoreat baseline | PASIScoreat Visit 7 | PASIScoreat Visit 10 | %reductionin PASI atVisit 7 | %reductionin PASI atVisit 10 |
|---|
| 1 | M | 67 | 27.6 | 21.2 | 15.7 | 23.19 | 43.12 |
| 2 | F | 60 | 23.2 | 7.5 | 0.3 | 67.67 | 98.71 |
| 3 | M | 80 | 40.5 | 9.8 | 3.4 | 75.80 | 91.60 |
| 4 | M | 65 | 29 | 11.3 | 5.2 | 61.03 | 82.07 |
| 5 | F | 55 | 32.6 | 6.2 | 0.6 | 80.98 | 98.16 |
| 6 | M | 65 | 28 | 8.7 | 1.1 | 68.93 | 96.07 |
| 7 | M | 80 | 12.5 | 8.4 | 3.3 | 32.80 | 73.60 |
| 8 | M | 70 | 29.9 | 7.2 | 1.2 | 75.92 | 95.99 |
| 9 | M | 84 | 30.5 | 8.5 | 2.6 | 72.13 | 91.48 |
| 10 | M | 74 | 23.4 | 9.1 | 3.3 | 61.11 | 85.90 |
| 11 | M | 72 | 19.2 | 10.2 | 2.2 | 46.88 | 88.54 |
| 12 | F | 65 | 27.2 | 7.5 | 2.8 | 72.43 | 89.71 |
| 13 | M | 70 | 24.8 | 8.7 | 3.2 | 64.92 | 87.10 |
| 14 | M | 75 | 42.6 | 7.9 | 2.4 | 81.46 | 94.37 |
| 15 | M | 68 | 28.5 | 10.3 | 4.2 | 63.86 | 85.26 |
| 16 | F | 61 | 26.1 | 9.3 | 2.2 | 64.37 | 91.57 |
| 17 | M | 70 | 21.3 | 6.4 | 1.6 | 69.95 | 92.49 |
| 18 | M | 68 | 25.8 | 7.2 | 1.4 | 72.09 | 94.57 |
| 19 | F | 65 | 27.5 | 10.2 | 3.2 | 62.91 | 88.36 |
| 20 | M | 76 | 17.5 | 10.4 | 3.3 | 40.57 | 81.14 |
Outcome of The Therapy
Psoriasis Area and Severity Index (PASI)
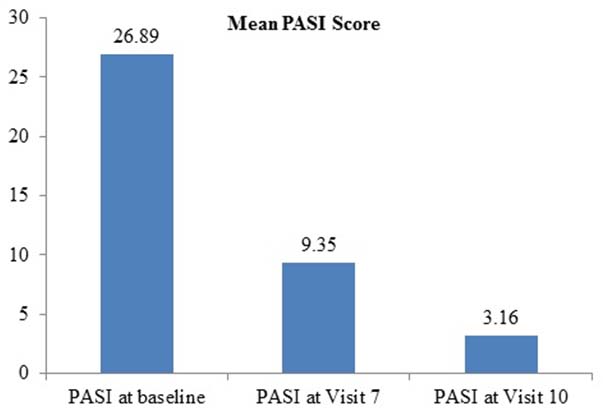
Clinically significant improvement in the psoriasis patients was observed as treatment of Itolizumab proceeded. The clinically visible improvement was noted in patients after completion of 3-4 infusions of Itolizumab treatment. Significant change in the mean PASI scores is depicted in [Table/Fig-2]. Further continuation of treatment exhibited significant improvement in lesion clearance [Table/Fig-3]. Albeit the degree of response varied from patient to patient, the mean PASI score was significantly reduced from baseline 26.89 to 9.35 and 3.16 at visit 7 and visit 10 respectively.
Effect of Itolizumab treatment on PASI scores of psoriasis patient.
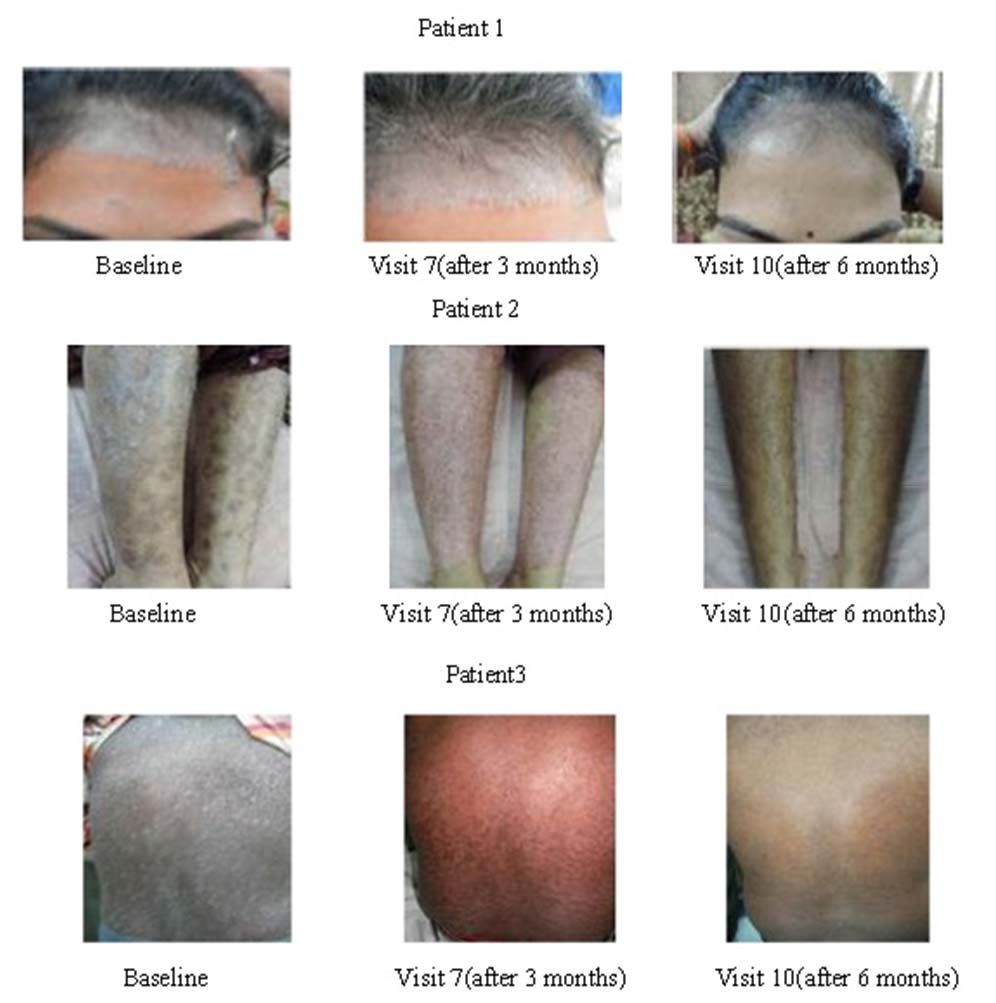
Patients images at baseline, and after receiving Itolizumab treatment at 3 months and 6 months.
Eighteen patients achieved PASI75 response after receiving 10 infusions of Itolizumab (at the completion of treatment). Out of 18 patients 4 patients had achieved PASI95 response and 10 patients had achieved PASI90 response. However, PASI 50 response was achieved in 1 patient after completion of treatment but one patient failed to achieve PASI 50.
Physician Global Assessment (PGA)
PGA in Itolizumab treated patients was assessed during each follow-up visit. The majority of patients were severe and moderate to severe as per PGA at baseline. After completion of treatment (10 infusions of Itolizumab), the majority of patients were mild to almost clear.
Adverse events
There was no any severe infusion reaction or severe infection experienced in patients during treatment period. No activation of latent tuberculosis was observed during and after the treatment.
Discussion
Management of psoriasis disease is rotational therapy from topical to biological. Conventional therapies are available for treatment of moderate to severe psoriasis but associated with cumulative toxicities [3]. The introduction of biological therapy has created a great impression on the duration of psoriatic treatment and enhancement of quality of life in psoriasis patients. Biological treatment for psoriasis has shown greater efficacy than conventional therapy due to highly specific immune activity. Therefore, they are considered as effective and safer [4]. The approved biological therapies are etanercept, infliximab, itolizumab, and adalimumab for the treatment of psoriasis in India [3,5]. Itolizumab is a humanized IgG1 monoclonal antibody (mAb) which acts by inhibition of CD6-mediated co-stimulation of T-cells and down-regulates production of multiple pro-inflammatory cytokines such as interferon, Interleukin-6 (IL-6) and Tumour Necrosis Factor (TNF) [6].
In the present case series, all patients exhibited significant improvement in PASI scores during Itolizumab treatment period. After completion of treatment, 18 patients achieved PASI75 score. PASI 75 or a reduction in baseline PASI score of >75%, is the standard used by FDA to assess the efficacy of a new psoriasis agent [7]. In this case series study, psoriasis patients included were previously on conventional systemic and topical treatment with unsatisfactory or no change in results. These patients showed a significant reduction in mean PASI score after treatment with Itolizumab from baseline 26.89 to 3.16 (visit 10). Thus, indicating the efficacy of itolizumab in psoriasis patient who were non-responsive to conventional therapies. In a phase II trial, Anand et al., reported a significant reduction in mean PASI score after itolizumab treatment from baseline to week 12 (22.32 to 6.23, p< 0.0001). Overall, 45 % of patients achieved the PASI 75 response at week 12 [8]. Similarly, in our case series, mean PASI score was significantly reduced from baseline to week 24 (visit 10) (26.89 to 3.16) and 90% patients achieved PASI 75 response.
In another study, Gupta A et al., reported that significant reduction in PASI score of a psoriasis patient after 12 weeks treatment of itolizumab (1.6 mg/Kg) from 33.5 to 1.4, and achieved PASI 90 response [9]. In our case series 10 patients had achieved PASI 90 response after the completion of itolizumab therapy. PGA score was assessed as severe and moderate to severe at baseline which was improved to mild to almost clear in the majority of patients after end of treatment period. Budamakuntla et al., reported that there was significant improvement in PGA scores from baseline 3 to 1 (Clear) after the end of itolizumab treatment [10]. The average patient remission period was of 4-6 months.
Krupashankar et al., reported the significant efficacy of itolizumab as compared to placebo, 27.0% in treatment arm A (0.4 mg/kg/wk itolizumab for 4 weeks, followed by 1.6 mg/kg every 2 weeks) (p = 0.0172 vs placebo), 36.4% in B (1.6 mg/kg every 2 weeks) (p = 0.0043 vs placebo), and 2.3% in the placebo achieved PASI 75 score at week 12. However, at week 28 PASI 75 score was comparable at 46.1%, 45.5%, and 41.9% for arm A, B, and placebo, respectively. The improvement in PASI 75 score in the placebo arm was observed after crossover to itolizumab at week 12 [11].
In a long-term study, at 1-year follow-up 52.5% patients in group 1P (itolizumab 1.6 mg/kg q12w) were good responders achieving long-term remission after controlled drug cessation while in group 1M (itolizumab 1.6 mg/kg q12w), 66.7% patients maintained PASI 75 representing good responders achieving long-term remission with maintenance therapy [12]. We observed average remission period of 6-8 months after the end of itolizumab treatment in our case series.
Common adverse effects such as infusion related reaction, pyrexia due to infections, pruritus, effects on blood were anticipated with itolizumab treatment [6]. Krupashankar et al., in their study reported that the incidences of adverse events were comparable between itolizumab groups and placebo group, and incidences of infections in itolizumab groups were not greater than placebo group [11]. They also concluded that the itolizumab is an effective and well-tolerated novel biological therapy in moderate to severe psoriasis. We also haven’t noticed any severe infusion reaction or severe infection. This indicates that itolizumab was safe and well-tolerated in psoriasis patients.
Conclusion
Itolizumab was found to be effective and safe in treatment of moderate-to-severe psoriasis patients.
[1]. European Medicines Agency. Evaluation of Medicines for Human Use. Cited from http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003329.pdf [Accessed on 12 Jul 16] [Google Scholar]
[2]. Smith CH, Anstey AV, Barker JN, Burden AD, Chalmers RJ, Chandler D, British Association of Dermatologists guidelines for use of biological interventions in psoriasis 2005Brit J Dermatol 2005 153(3):486-97. [Google Scholar]
[3]. Mustafa AA, Al-Hoqail IA, Biologic systemic therapy for moderate-to-severe psoriasis: A reviewJ Taibah Univ Med Sci 2013 8(3):142-50. [Google Scholar]
[4]. Sivamani RK, Correa G, Ono Y, Bowen MP, Raychaudhuri SP, Maverakis E, Biological Therapy Of PsoriasisIndian J Dermatol 2010 55(2):161-70. [Google Scholar]
[5]. List of Recombinant DNA based Drugs Approved in the Country (Form-45) 1st Jan 2010 - 31st Dec 2013. Cited from http://cdsco.nic.in/writereaddata/FORM45%20updated.pdf [Accessed on 20 Apr 16] [Google Scholar]
[6]. Menon R, David BG, Itolizumab – a humanized anti-CD6 monoclonal antibody with a better side effects profile for the treatment of psoriasisClin Cosmet Investig Dermatol 2015 8:215-22. [Google Scholar]
[7]. Veena RM, Satheesh BC, Sumathy TK, To evaluate the efficacy of Anthralin with and without coaltar in short contact therapy of mild to moderate psoriasis- A randomized double blind controlled studyInt J Cur Res Rev 2015 7(4):68-71. [Google Scholar]
[8]. Anand A, Assudani D, Nair P, Krishnamurthy S, Deodhar S, Arumugam A, Safety, efficacy and pharmacokinetics of T1h, a humanized anti-CD6 monoclonal antibody, in moderate to severe chronic plaque psoriasis results from a randomized phase II trialJ Immunol 2010 184:96.13 [Google Scholar]
[9]. Gupta A, Sharma YK, Deo K, Kothari P, Severe recalcitrant psoriasis treated with itolizumab, a novel anti-CD6 monoclonal antibodyIndian J Dermatol Venereol Leprol 2016 82(4):459-61. [Google Scholar]
[10]. Budamakuntla L, Madaiah M, Sarvajnamurthy S, Kapanigowda S, Itolizumab provides sustained remission in plaque psoriasis: a 5-year follow-up experienceClin Exp Dermatol 2015 40(2):152-55. [Google Scholar]
[11]. Krupashankar DS, Dogra S, Kura M, Saraswat A, Budamakuntla L, Sumathy TK, Efficacy and safety of itolizumab, a novel anti-CD6 monoclonal antibody, in patients with moderate to severe chronic plaque psoriasis: results of a double-blind, randomized, placebo-controlled, phase-III studyJ Am Acad Dermatol 2014 71(3):484-92. [Google Scholar]
[12]. Dogra S, D S K, Budamakuntla L, Srinivas CR, Khopkar U, Gupta S, Long-term efficacy and safety of itolizumab in patients with moderate-to-severe chronic plaque psoriasis: A double-blind, randomized-withdrawal, placebo-controlled studyJ Am Acad Dermatol 2015 73(2):331-33.e1. [Google Scholar]