Pulmonary Hamartoma Mimicking Malignancy: A Cytopathological Diagnosis
Manjari Kishore1, Prajwala Gupta2, Preeti3, Desh Deepak4
1 Senior Resident, Department of Pathology, Postgraduate Institute of Medical Education and Research, Dr. RML Hospital, New Delhi, India.
2 Associate Professor, Department of Pathology, Postgraduate Institute of Medical Education and Research, Dr. RML Hospital, New Delhi, India.
3 Junior Resident, Department of Pathology, Postgraduate Institute of Medical Education and Research, Dr. RML Hospital, New Delhi, India.
4 Chief Medical Officer, Department of Respiratory Medicine, Postgraduate Institute of Medical Education and Research, Dr. RML Hospital, New Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Prajwala Gupta, Room no. 302, OPD Block Dr. RML Hospital, Baba Kharag Singh Marg, New Delhi-110001, India.
E-mail: prajwala2000@yahoo.com
Pulmonary Hamartomas (PH) are benign tumour-like lesions of lung with an uncommon occurrence and pose a diagnostic challenge on chest radiograph. Fine Needle Aspiration Cytology (FNAC) can lead to a definitive diagnosis as well as distinguishes these from malignant lung mass. Most of the patients are asymptomatic and incidentally detected on routine chest radiographs. We report a case of pulmonary hamartoma where the patient was symptomatic and a possibility of malignant neoplasm was considered until the FNAC concluded the diagnosis.
FNAC, Hamartoma, Lung, Neoplasm, Radiology
Case Report
A 53-year-old male presented to medicine outpatient department with complaints of cough with sputum for last four months. The patient gave a history of chronic smoking and change in voice. However, there was no history of fever, dyspnea, haemoptysis, dysphagia, loss of appetite and weight loss. On general examination, there was absence of pallor, cyanosis, clubbing, oedema and any peripheral lymphadenopathy. On examination of the respiratory system mild left sided ronchi was noted on auscultation, however, bilateral air entry was normal. Rest of the systems examined did not reveal any abnormality.
Chest X-ray PA view showed a large well defined mass in the left upper lobe of lung with interspersed tiny areas of calcification [Table/Fig-1a]. Fibreoptic bronchoscopy did not reveal any abnormal findings. Bronchial aspirate and sputum examination were negative for malignant cells.
a) Chest X-Ray PA view showing a large solitary well defined mass in the left upper lobe of lung (arrow). (b) CECT chest showing a well-defined heterogeneously enhancing soft tissue parenchymal mass with interspersed areas of fat and dense calcification in the upper lobe of left lung (arrow).
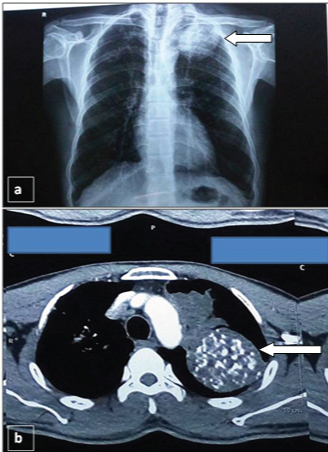
Complete blood count revealed mild anaemia (Haemoglobin- 9.2 gm/dl) and peripheral smear showed normocytic normochromic red blood cells whereas other parameters were within normal range. An increase in Erythrocyte Sedimentation Rate (ESR) (25 mm at the end of 1st hour) was noted. Routine biochemical and urine examination were unremarkable. Clinically, provisional diagnosis of carcinoma lung was considered. Further, Contrast Enhanced Computed Tomography (CECT) of neck revealed a mass lesion in left upper lobe of lung. CECT chest showed well defined heterogeneously enhancing soft tissue density measuring 59x58x54mm with dense calcification and foci of fat within, in the upper lobe of left lung [Table/Fig-1b]. However, overlying ribs were normal. Also, noted was another heterogeneously enhancing soft tissue density mass with irregular margin, measuring 50X36X29 mm, in apico-posterior segment of upper lobe of the left lung, which represented collapsed consolidated portion of lung. Confluent and discrete centrilobular nodules and adjacent emphysematous changes along with fibro-bronchiectatic changes were also noted in the vicinity of the above mentioned lesions.
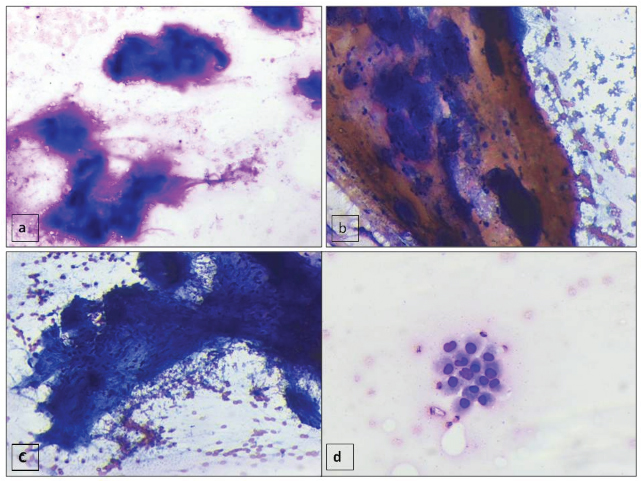
Computed Tomography (CT) guided FNAC from the larger soft tissue mass was done. Subsequent smears prepared were fixed in 95% ethyl alcohol and air dried followed by staining with Papanicolaou and Giemsa stain respectively. Smears examined showed numerous fragments of fibrillary metachromatic material (chondromyxoid) [Table/Fig-2a,b]. Occasional matrix material revealed embedded bland spindled cells [Table/Fig-2c]. Also, noted were few groups and occasional singly scattered round to oval cells with eccentric nuclei and scant to moderate amount of cytoplasm (bronchial cells) in a hemorrhagic background [Table/Fig-2d]. However, fat cells were not noted in the cytological smears.
(a,b) FNAC smears showing fragments of metachromatic fibrillary (chondromyxoid) material (a- Giemsa X 200, b - Pap X 100). (c) Fragment of bland spindled cells embedded in the myxoid stromal material (Pap, X100). (d) Occasional cluster of round to oval benign bronchial epithelial cells with eccentric nuclei and scant to moderate amount of cytoplasm (Giemsa, X200) were also noted.
In view of the cytomorphological features and radiological details, a diagnosis of pulmonary hamartoma was made. Further, the patient was advised surgical excision in view of the large mass; however, patient declined surgery. He was treated for Chronic Obstructive Pulmonary Disease (COPD) and is presently under follow-up for past six months with no change in the radiographic size.
Discussion
Pulmonary hamartomas (PH) are abnormal mixture of normal developmental components, with incidence of 0.23-0.32%. Although of uncommon occurrence, these happen to be the most common benign lesion of lung and account for approximately 6% of all the solitary pulmonary nodules [1]. These solitary lesions seen on chest radiographs occur due to various causes like primary malignancies, metastasis, granuloma or other benign tumours [2]. PHs are usually small, less than 4cm to 8cm [2]. The patients are usually asymptomatic when the lesion is in peripheral parenchyma. Endobronchial location causes obstructive symptoms and may also present with chest tightness or repeated respiratory tract infection [3,4]. In the present case, the patient had a peripheral lung hamartoma but was symptomatic with presence of chronic cough. Moreover, the history of smoking with change in voice and the chest radiograph findings were favoring more of a carcinoma than a hamartoma. Lien et al., reported similar findings with history of smoking and cough in 52% and 21% of the patients respectively [3].
CT findings are not always diagnostic and the characteristic popcorn calcification (linear, nodular, irregular shaped calcifications) is seen in 10-30% of cases along with the heterogeneous appearance [5]. Therefore, the role of radiology in differentiating these lesions from other causes of solitary nodule is limited. CT findings in our case were favoring hamartoma showing dense calcification and heterogeneous areas. For a conclusive diagnosis of hamartoma and to exclude malignancy, cytological or histopathological examination is required [1,6]. Endobronchial hamartomas can be diagnosed by flexible fibreoptic bronchoscopic transbronchial aspiration or biopsy. FNAC or wedge resection is sufficient for peripherally located lesions [2,7].
FNAC yields often scant aspirate because of the dense nature of the lesion. The most dependable finding in FNAC is the presence of mesenchymal matrix resembling pleomorphic adenoma of the parotid gland [8]. This sparsely cellular material is fibrillary and contains few scattered, benign, spindled or stellate mesenchymal cells which were noted in the present case also. These matrix material are lightly cyanophilic in Pap-stained smears and brightly metachromatic with Giemsa stain. These lesions also contain both epithelial component like benign bronchial cells as was seen in our case. It is mentioned in literature that if pulmonary hamartoma can be recognized in FNA sample confidently, patient can be advised to forego surgical excision [8].
Hughes JH et al., reviewed a total of 19 FNAC specimens of PH and reported 78% specificity of FNAC for making the correct interpretation of this benign entity [7]. They also noted that the sensitivity for correctly diagnosing PH on cytology was only 26% [7]. The high false positive rate was explained because of subtle presence of the mesenchymal component on Papanicolaou stained smears and secondly the presence of very cellular epithelial component led to misdiagnosis of low grade neoplasms like carcinoid tumour and other primary lung carcinomas. Hence, these warrant the cytologists to be aware of the potential false-positive interpretations that can occur in cytological examination of pulmonary hamartoma.
Histologically, hamartomas consist mostly of mesenchymal tissues, shows various combination of cartilage, adipose tissue, smooth muscle and rarely bone [9]. These lesions can be advised for surgical resection, like enucleation, wedge resection, lobectomy, or rarely pneumonectomy, depending on size and location of tumour along with various morphological patterns [9,10]. Proper radiological follow up is essential in these cases, to check the stability in size of tumour. There are reports of cases of malignancy arising from pre-existing PH [8–11].
Conclusion
We emphasize that in a deceptive clinical presentation and considering the rarity of pulmonary hamartoma, FNAC findings can be a quick and reliable diagnostic tool in conjunction with radiological findings for early diagnosis.
[1]. Wiatrowska BA, Yazdi HM, Matzinger FR, MacDonald LL, Fine needle aspiration biopsy of pulmonary hamartomas. Radiologic, cytologic and immunocytochemical study of 15 casesActa Cytol 1995 39:1167-74. [Google Scholar]
[2]. Pollock AB, Al Hasan M, Roy TM, Byrd RP, Pulmonary hamartoma-an algorithmic approach to the diagnosis and managementClin Pulm Med 2008 15:35-39. [Google Scholar]
[3]. Lien Y.C, Hsu H.S, Li WY, Wu Y.C, Hsu W H, Wang L.S, Pulmonary hamartomaJ Chin Med Assoc 2004 67:21-26. [Google Scholar]
[4]. Whyte RI, Donington JS, Hamartomas of the lungSemin Thorac Cardiovasc Surg 2003 15:301-04. [Google Scholar]
[5]. Ganti S, Milton R, Davidson L, Anikin V, Giant pulmonary hamartomaJ Cardiothorac Surg 2006 1:19 [Google Scholar]
[6]. Wood B, Swarbrick N, Frost F, Diagnosis of pulmonary hamartoma by fine needle biopsyActa Cytol 2008 52:412-17. [Google Scholar]
[7]. Hughes JH, Young NA, Wilbur DC, Renshaw AA, Mody DR, Fine-needle aspiration of pulmonary hamartoma: A common source of false-positive diagnoses in the College of American Pathologists Inter laboratory comparison program in nongynecologic cytologyArch Pathol Lab Med 2005 129:19-22. [Google Scholar]
[8]. Lee BJ, Kim HR, Cheon GJ, Koh JS, Kim CH, Lee JC, Squamous cell carcinoma arising from pulmonary hamartomaClin Nucl Med 2011 36:130-31. [Google Scholar]
[9]. Ribet M, Jaillard-Thery S, Nuttens MC, Pulmonary hamartoma and malignancyJ Thorac Cardiovasc Surg 1994 107:611-14. [Google Scholar]
[10]. Cosio BG, Villena V, Echave-Sustaeta J, de Miguel E, Alfaro J, Hernandez L, Endobronchial hamartomaChest 2002 122:202-05. [Google Scholar]
[11]. Umashankar T, Devadas AK, Ravichandra G, Yaranal PJ, Pulmonary hamartoma: Cytological study of a case and literature reviewJ Cytol 2012 29:261-63. [Google Scholar]