Large Cell Calcifying Sertoli Cell Tumour of Testis-A Rare Case Report
Harresh Kumar1, Nadeem Tanveer2, Natasha Gupta3, Kiran Mishra4
1 Postgraduate Student, Department of Pathology, University College of Medical Sciences, Dilshad Garden, Delhi, India.
2 Associate Professor, Department of Pathology, University College of Medical Sciences, Dilshad Garden, Delhi, India.
3 Assistant Professor, Department of Radiology, University College of Medical Sciences, Dilshad Garden, Delhi, India.
4 Director and Professor, Department of Pathology, University College of Medical Sciences, Dilshad Garden, Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Nadeem Tanveer, Associate Professor, Department of Pathology, University College of Medical Sciences, Delhi-110095, India.
E-mail: ntobh104@yahoo.co.in
Sertoli cell tumours of testes are classified into sertoli cell tumour NOS (not otherwise specified), sclerosing variant and large cell calcifying variant. So far, 90 cases of the large cell calcifying variant have been reported in literature. We describe a rare case of inhibin negative locally invasive large cell calcifying sertoli cell tumour of testis. A 62-year-old man presented with complaints of pain and swelling in right scrotum for 8 months. Ultrasound revealed a right testicular mass with internal vascularity and calcification. Gross examination of right inguinal orchiectomy specimen showed firm to hard mass with yellow areas and calcification seen on cut section. Microscopy revealed a tumour in the testis infiltrating the epididymis and rete testis and reaching up to the skin. Tumour cells were arranged in the form of solid nests, tubules and cords with neutrophilic stromal infiltrate and calcification. Tumour cells had abundant clear to eosinophilic cytoplasm, round nucleus with vesicular chromatin and conspicuous nucleoli. On immunohistochemistry, tumour cells were positive for pan cytokeratin, Epithelial Membrane Antigen (EMA), S-100 protein, desmin, vimentin, neuron specific enolase, and chromogranin. However, it was negative for inhibin alpha, OCT4, CD10, CD99, Melan A. Inhibin negative large cell calcifying sertoli cell tumour is a rare entity.
Calcification, Inhibin, Orchiectomy
Case Report
A 62-year-old man was admitted to the hospital with complaints of right scrotal swelling and pain for eight months. On physical examination of the patient, cardiovascular system, respiratory system, per abdomen was normal. Blood pressure was 135/80 mm Hg, pulse rate was 84 per minute and respiratory rate was 16 per minute. Patient had no history of hypertension, diabetes mellitus or any other disease. There were no hyperpigmented macules on lips, gynaecomastia, impotence, anaemia, lymphadenopathy or any other tumour except for a right sided testicular swelling. Swelling was hard, non tender and fixed to the scrotal skin and measured 8x3x2cm. Swelling showed no translucency and there was no hydrocele. Left side testis was normal. Clinical diagnosis was testicular tumour possibly malignant.
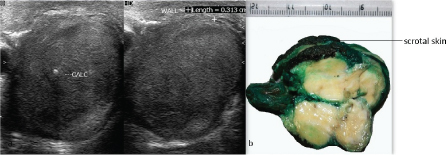
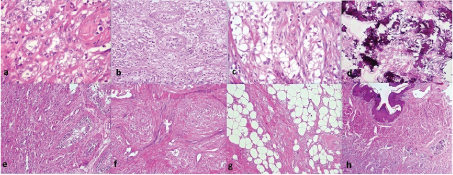
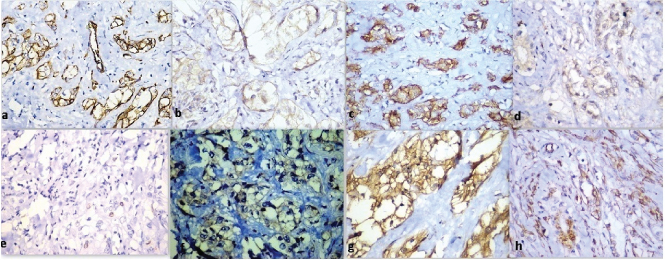
Ultrasound revealed a right testicular mass with internal vascularity and calcification [Table/Fig-1a]. Left side testis, bilateral kidneys, prostate and urinary bladder were normal on ultrasonography. Ultrasonogram did not show intrabdominal lymphadenopathy. Lab investigations showed haemoglobin 14.1 gm/dl, TLC 7800 per cu.mm, platelets 160000 per cu.mm, random blood sugar 105 mg/dl, serum sodium was 142 mEq/L, potassium was 3.9 mEq/L. Serum lactate dehydrogenase was 200 U/L (normal 115-221 U/L), serum alpha feto protein was 3.4 ng/ml (Normal 0-8.5 ng/ml) and serum beta HCG was 1.1 mIU/ml (normal <5 mIU/ml). Serum testosterone, serum prostate specific antigen levels and serum estrogen levels were within normal limits. High inguinal orchiectomy specimen of right testis measuring 8x3x2 cm and skin attached to upper pole of testis was received. The tumour measured 6x3x1.5cm. The cut section was yellowish with fleshy areas. Numerous areas of calcification were also seen [Table/Fig-1b]. Testicular tissue was almost completely replaced by the tumour. Tumour was adherent to the scrotal skin. No necrosis or haemorrhage was identified on gross examination. Microscopic examination revealed uncircumscribed, unencapsulated tumour with tumour cells present in the form of solid nests, tubules and cords [Table/Fig-2a]. Tumour cells had an abundant eosinophilic to clear cytoplasm, round to oval nucleus with vesicular chromatin and conspicuous to prominent nucleoli [Table/Fig-2a]. Tumour also showed an intratubular component with thickened basement membranes [Table/Fig-2b]. Stroma was fibrous with neutrophilic infiltrate in many areas [Table/Fig-2c]. Calcification was prominent [Table/Fig-2d]. Tumour infiltrated epididymis, rete testis and reached upto the dermis of scrotal skin [Table/Fig-2e-h]. There was no mitosis, necrosis, reinke crystals or lipofuscin or vascular invasion. On immunohistochemistry, tumour was positive for pan cytokeratin, Epithelial Membrane Antigen (EMA), S-100 protein, desmin, vimentin, neuron specific enolase, focally for calretinin and chromogranin [Table/Fig-3]. However, it was negative for inhibin alpha, OCT4 (to exclude germ cell tumours), melan A, CD99 (to exclude leydig cell tumour), CD10 (to exclude both leydig cell tumour and clear cell RCC), Wilms tumour protein-1 (WT-1) and D2-40 (podoplanin) (to rule out adenomatoid tumour). Synaptophysin was also negative. Ki-67 index was 4%. Final diagnosis of large cell calcifying sertoli cell tumour was made. Pathological staging of tumour was T4N0M0. Clinical staging was stage 1b. Patient was kept under follow up on view of extratesticular invasion. The follow up has been uneventful at 6 months.
(a) Ultrasound of the testis showing tumour with calcification. (b) Gross photograph shows tumour is yellowish white in colour with fleshy areas along with attached scrotal skin.
(a) Tumour cells arranged as tubules with eosinophilic cytoplasm with intratubular component (H & E, 40X); (b & c) Tumour cells arranged as nests with clear cytoplasm and neutrophilic stromal infiltrate (H & E, 20X); (d) Calcification was prominent (H & E, 10X); (e-h) Tumour infiltrated epididymis, rete testis and reached upto the dermis of scrotal skin.(H & E, 10X).
Immunohistochemistry (positive markers) (a) Pan cytokeratin (10X); (b) Desmin (40X); (c) Epithelial membrane antigen (10X); (d) S-100 protein (40X); (e) Ki-67 (10X); (f) chromogranin (40X); (g) Neuron Specific Enolase (40X); (h) Vimentin (10X).
Discussion
Large cell calcifying sertoli cell tumour was first described in 1980 by Proppe and Scully [1]. Ninety cases have been reported in literature till date [2]. Sex-cord stromal tumours form 4% and sertoli cell tumours comprise 1% of total testicular tumours. Variants of sertoli cell tumours are sertoli cell tumour Not Otherwise Specified (NOS), sclerosing and large cell calcifying variant [Table/Fig-4]. Only 17% of these tumours are malignant and few locally invasive tumours have been described [3,4]. Inhibin negativity does not rule out the diagnosis of sertoli cell tumour [5].
Sex cord stromal tumours of testis-comparison of histomorphological features.
| Sertoli cell tumour NOS | Sclerosing sertoli cell tumour | Large cell calcifying sertoli cell tumour | Leydig cell tumour |
|---|
| Tubules | Tubules | Tubules | Sheets/tubules |
| Fibrous stroma | Dense collagenous stroma | Fibrous stroma | Fibrous stroma |
| Pale to clear cytoplasm | Pale cytoplasm | Abundant eosinophilic to clear cytoplasm | Abundant eosinophilic cytoplasm |
| Round to oval uniform nuclei | Small euchromatic nuclei | Round nuclei with or without prominent nucleoli | Round nuclei with prominent nucleoli |
| | Neutrophilic stromal infiltrate. | Reinke crystalloids |
| | Calcification | Lipofuschin |
Large cell calcifying sertoli cell tumour, is commonly seen in young patients and may be associated with Carney’s syndrome and Peutz-Jeghers syndrome [6]. Tumours associated with clinical syndromes are usually bilateral and multicentric. Malignant tumours are usually sporadic but occasional malignancy has been reported in patient with Carney syndrome and Peutz-Jeghers syndrome. Malignant tumours are usually unilateral and solitary [7,8]. Our patient had a unilateral tumour without familial syndrome.
In a typical tumour, cells are arranged in the form of solid nests, tubules and cords. Neutrophilic stromal infiltrate is seen. Intratubular growth component is common. Calcification can be seen in the form of ossification, amorphous masses, and concentric masses. Tumour cells have abundant eosinophilic cytoplasm with round nucleus with or without prominent nucleoli [9]. Cells have abundant clear cytoplasm [10]. Our case had all these features with cells with clear to eosinophilic cytoplasm. Of a total of 90 reported cases, sixteen cases of large cell calcifying sertoli tumour have been reported as malignant [2]. Malignant behaviour is associated with large size (size>4cm), necrosis, increased mitotic activity, atypia, and vascular invasion. A tumour with any two of these criteria qualifies for malignancy [6,11]. In our case, tumour size was greater than 5 cm, but other features of malignancy were absent. However in view of rarity of the tumour and presence of extratesticular invasion the patient was placed on regular follow up.
Large cell calcifying sertoli tumour is immunopositive for inhibin alpha, calretinin, neuron specific enolase, desmin, S100 protein, and vimentin. Inhibin-alpha is positive in 90% of sertoli cell tumours [3]. Ki-67 index was 4% in our case. Malignant tumours generally have index above 30% [3]. Our case was strongly positive for all the above except inhibin alpha. Calretinin and chromogranin showed focal weak positivity. Inhibin negative sertoli cell tumours have been described and negativity for this marker does not exclude the diagnosis of this tumour [12]. Positivity of neuroendocrine markers can be explained by the fact that sex cord stromal cells (sertoli cells, leydig cells, granulosa cells) probably are members of disperse neuroendocrine system [5]. In the differential diagnoses, leydig cell tumour, adenomatoid tumour and metastatic renal cell carcinoma were considered [8,13]. Neutrophilic stromal infiltrate and calcifications as seen in our case, are not seen in metastatic clear cell renal cell carcinoma, adenomatoid tumour and Leydig cell tumour. Reinke crystals, absence of intratubular growth favour leydig tumour. In our case, reinke crystals were absent and intratubular growth was seen [3]. Findings favouring clear cell renal cell carcinoma include lack of a distinct mass on gross examination, bilaterality, conspicuous intertubular growth, and prominent intralymphatic spread. These findings were absent in our case [14]. Immunohistochemistry was negative for CD10 which ruled out clear cell RCC, while Inhibin, CD10, Melan A were negative, ruling out leydig cell tumour. Immunohistochemistry was negative for WT-1, D2-40 which ruled out adenomatoid tumour. Radical inguinal orchiectomy is the treatment of choice for this tumour. Retroperitoneal lymph node dissection is necessary if lymph nodes are involved by tumour in malignant cases [15]. Patients with gynaecomastia can be managed using pharmacotherapy [6].
Conclusion
Large cell calcifying sertoli tumour is benign to intermediate grade in most of the cases. Rare malignant cases have been described. Tumour can be inhibin negative. It has associations with Carney’s complex and Peutz-Jeghers syndrome. Investigations should be done to rule out familial syndromes in young patients with this tumour.
[1]. Chang B, Borer JG, Tan PE, Diamond DA, Large-cell calcifying Sertoli cell tumour of the testis: case report and review of the literatureUrology 1998 52(3):520-22. [Google Scholar]
[2]. Lan T, Zhuang H, Zhang H, Case Report Large cell calcifying sertoli cell tumour of the testis: a case reportInt J Clin Exp Pathol 2016 9(3):3972-77. [Google Scholar]
[3]. Ulbright TM, Emerson RE, Neoplasms of the testis. Bostwick DG, Cheng L (eds)Urologic surgical pathology 2013 3rd edPhiladephiaElsevier:738-828. [Google Scholar]
[4]. Song DH, Jeong SM, Park JT, Yun GW, Kim BK, Lee JS, Large cell calcifying sertoli cell tumour of the testis: a case study and review of the literatureKorean J Pathol 2014 48(1):50-3. [Google Scholar]
[5]. Kuroda N, Senzaki T, Yamasaki Y, Miyazaki E, Hayashi Y, Toi M, Sertoli cell tumour of the testis (not otherwise specified) with the expression of neuroendocrine markers and without the expression of inhibin-alphaPathol Int 2004 54(9):719-24. [Google Scholar]
[6]. Gourgari E, Saloustros E, Stratakis CA, Large-cell calcifying Sertoli cell tumours of the testes in pediatricsCurr Opin Pediatr 2012 24(4):518-22. [Google Scholar]
[7]. Berney MD, Ulbright MT, Difficult or Newly Described Morphologic Entities in Testicular Neoplasia. In: Magi-Galluzzi C, Przybycin GC, editorsGenitourinary Pathology: Practical Advances 2015 New York, NYSpringer New York:471-81. [Google Scholar]
[8]. Guha P, Sarkar D, Ray A, Thakur I, Mukherjee S, Chatterjee SK, Malignant Large Cell Calcifying Sertoli Cell Tumour of Testis with Skip Metastasis to Lung Presented With Peutz-Jeghers SyndromeTanaffos 2012 11(4):63 [Google Scholar]
[9]. Ulbright TM, Young RH, Testicular and paratesticular tumours and tumour-like lesions in the first 2 decadesSemin Diagn Pathol 2014 31(5):323-81. [Google Scholar]
[10]. Petersson F, Bulimbasic S, Sima R, Michal M, Hora M, Malagon HD, Large cell calcifying Sertoli cell tumour: a clinicopathologic study of 1 malignant and 3 benign tumours using histomorphology, immunohistochemistry, ultrastructure, comparative genomic hybridization and polymerase chain reaction analysis of the PRKAR1A geneHum Pathol 2010 41(4):552-59. [Google Scholar]
[11]. Ulbright TM, Amin MB, Young RH, Atlas of Tumour Pathology, Tumours of the Testis, Adnexa, Spermatic Cord, and Scrotum 1999 Washington, D.C.AFIP Press:12 [Google Scholar]
[12]. McCluggage WG, Shanks JH, Whiteside C, Maxwell P, Banerjee SS, Biggart JD, Immunohistochemical study of testicular sex cord-stromal tumours, including staining with anti-inhibin antibodyAm J Surg Pathol 1998 22(5):615-9. [Google Scholar]
[13]. Ulbright TM, Berney DM, Testicular and Paratesticular tumours. Mills SE, Greenson JK, Hornick JL, Longacre TA, Reuter VE (eds)Sternberg’s Diagnostic Surgical Pathology 2015 6th edPhiladephiaLippincott Williams & Wilkins:2175-2245. [Google Scholar]
[14]. Ulbright TM, Young RH, Metastatic carcinoma to the testis: A clinicopathologic analysis of 26 nonincidental cases with emphasis on deceptive featuresAm J Surg Pathol 2008 32(11):1683-93. [Google Scholar]
[15]. Halat SK, Ponsky LE, MacLennan GT, Large cell calcifying Sertoli cell tumour of testisJ Urol 2007 177(6):2338 [Google Scholar]