Blood Contamination of the Small Bore Peripheral Intravenous Catheter in Neonates
Vani Krishnamurthy1, Srinivasa Murthy Doreswamy2, Sushma Krishnagowda3
1 Assistant Professor, Department of Pathology, JSS Medical College, JSS University, Mysuru, Karnataka, India.
2 Professor, Department of Pediatrics, JSS Medical College, JSS University, Mysuru, Karnataka, India.
3 Assistant Professor, Department of Pediatrics, JSS Medical College, JSS University, Karnataka, Mysuru, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Vani Krishnamurthy, 70, Prakruthi, BEML 2nd Stage, Rajarajeshwari Nagara, Mysuru-570026, Karnataka, India.
E-mail: vanidrsri@gmail.com
Introduction
Peripheral Intravenous Catheters (PIV) are extensively used in sick neonates for administration of medicines and nutrition. When these PIVs are used on intermittent basis, they are flushed with saline in order to keep the hub of the catheter free from blood. Presence of blood in the hub of the catheter can be potentially dangerous as it could facilitate infection.
Aim
The aim of this study was to find the magnitude of blood contamination of PIV catheter hub after routine flushing.
Materials and Methods
We measured the volume of 24 g PIV by filling it with saline and thereby measuring its volume. The PIVs which were in situ for at least 6 hours and removed were used for this study. These catheters were flushed with 0.2 ml of saline and the RBC count was calculated.
Results
A total of 94 PIVs were studied, out of which 84% showed blood tinged residual flush and 15% of them had visible blood clot. All (100%) of the catheter studied showed RBCs on microscopic examination. The median RBC count was 36960/cu mm and the interquartile range was 10000 – 113920/cu mm. The highest RBC count was 2080000/cu mm.
Conclusion
Blood contamination of the small bore PIVs after flushing is universal in neonates.
Contamination, Newborn, Peripheral cannula
Introduction
Peripheral Intravenous Catheters (PIV) are extensively used in sick neonates for administration of medications and nutrients. Use of these PIVs can be either continuous or intermittent depending on the clinical need of the baby. When these PIVs are used intermittently, they are flushed with solutions like normal saline or heparin saline in order to provide a fluid lock and prevent blockade. The technique of flushing venous catheter is not uniform in all neonatal units [1]. The common method of flushing in our neonatal unit is to push 1 ml of normal saline through the needleless venous access bung – Bionector – (Vygon, France). However, a common observation is that there is some blood-tinged solution still left at the end of flush [Table/Fig-1]. Such a solution, which contains blood can potentially act as a good medium for the bacteria to grow. Blood contamination of the hub is known to predispose infection with central catheters [2].
Showing Blood tinge in the catheter hub after flushing.
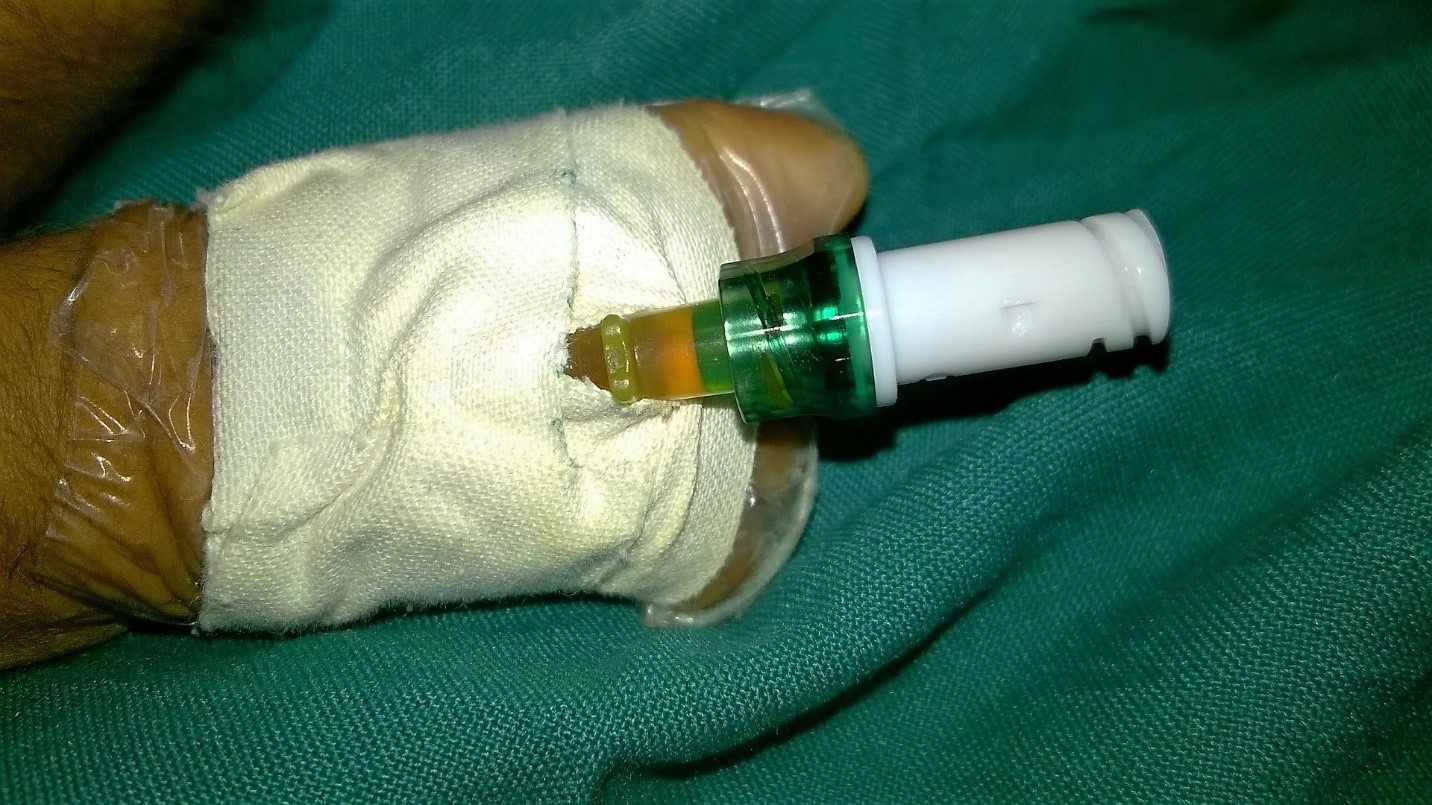
Thrombophlebitis due to peripheral IV cannula is a recognized entity in neonates [3]. Keeping the catheter hub clean without blood contamination has shown to decrease the blood stream infection in children with central venous catheters [2,4]. In view of this, CDC has produced clear guidelines for the technique of flushing central venous catheters [5]. However, similar guidelines are not available for peripheral IV catheters, particularly in neonates.
Though blood residue after flushing the PIV is a common observation, there is no systematic and scientific study conducted on blood contamination of the PIVs after the routine normal saline flush in neonatal population. We believe scientific information on this issue will be helpful in devising policies for flushing the PIV, which has a potential to reduce catheter related infections in neonatal intensive care units.
The primary objective of this study was to estimate the proportion of PIV catheters showing blood contamination after routine normal saline flushing in neonatal population. Secondary objectives were to calculate the Red Blood Cell (RBC) burden in the contaminated PIV catheters and to assess the correlation between the burden of RBC and duration of PIV catheter stay before discarding.
Materials and Methods
This was a prospective observational descriptive study conducted in Department of Neonatology and Pathology of a tertiary care hospital (JSS hospital, Mysuru) in the month of January and February 2016. The PIVs, which were removed and discarded after their clinical utility were used for this study. Inclusion criteria were: a) Peripheral intravenous catheter of 24 g; b) Has been in situ for at least 6 hours; c) No continuous infusion through the PIV for last 6 hours; d) No local infection at the site of PIV insertion.
We conducted a pilot study with 10 samples and noted that 90% of the sample demonstrated visible blood in the catheter after flushing [Table/Fig-1]. Considering the prevalence to be 90%, alpha of 0.05 and confidence levels of 89%, we needed 92 samples. Categorical variables were reported as proportions. Continuous variables were analysed for their distribution and summarized as mean and standard deviation or median and interquartile range as appropriate. Microsoft excel 2013 was used for data tabulation and ananlysis.
The experiment consisted of three steps: Step 1 was to estimate the volume of the PIV catheter that was done at the beginning of the study and only once; Step 2 was to obtain the residual blood from the hub of the catheter by flushing; and Step 3 was counting the RBCs in the flush thus obtained.
Step 1 - The volume of the PIV (24 Gauge) was measured as follows. PIV 24 G was completely filled with saline using a syringe. The plunger of the syringe was operated till the amount of saline left in the syringe was 0.5 ml (injected volume). The catheter tip was positioned into a Pediatric EDTA vacutainer. 0.5 ml of saline was now pushed through the catheter which was followed by 1ml air flush to empty the catheter. The saline collected in the vacutainer was aspirated into another syringe and the volume was noted (final observed volume). The volume of the catheter was calculated as final observed volume minus injected volume (0.5 ml). The experiment was repeated three times and the average volume calculated. We measured the volume of the PIV catheter to be 0.2 ml. Finally, this volume was used to flush the experimental catheters.
Step 2 - Once the catheter was removed from the patient, it was held over an EDTA vacutainer with tip pointing into the vacutainer. 0.2 ml of saline was pushed through the catheter. This was followed by 1 ml air flush to empty the catheter. The total volume obtained in the vacutainer was the volume of the catheter plus the volume of saline flush which was equal to 0.4 ml. This resulted in a dilution factor of 2 of the residual flush. Then, the solution in the vacutainer was labelled with the serial number and transported to the Department of Pathology.
Step 3 - RBC count was performed by standard technique using Neubauer chamber within 30 minutes of receipt of the sample. To obtain the actual value of residual blood in the hub of the PIV catheter, the value was multiplied by dilution factor 2. The values were tabulated in Microsoft excel 2013.
Results
We included 106 catheters, which were to be discarded. One of the catheter was completely contaminated with blood during removal and hence not included for further analysis. Four samples were lost in the transit and seven were analysed after 12 hours of collection. These values were considered unreliable due to hemolysis and hence, were not included for final analysis. Thus, a total of 94 samples were finally analysed.
A total of 56% of PIV catheters were removed from term babies and 44% from preterm babies. The minimum duration of PIV catheter in place was 8 hours and maximum duration was 120 hours before we studied them. The minimum time duration between the last routine flush and study was one hour and maximum was 24 hours. Baseline characteristics are depicted in [Table/Fig-2].
Baseline characteristics.
| Parameter | Number (%) |
|---|
| Male (n, %) | 60 (63.8) |
| Female (n, %) | 34 (36) |
| Gestation in weeks (Mean, SD) | 35.9 (2.9) |
| Birth weight in grams (Mean, SD) | 2428 (688) |
| Term gestation (n, %) | 53 (56) |
| Preterm gestation (n, %) | 41(44) |
| Catheter in situ (Min - Max) hours | 8 - 120 |
| Catheter in situ (Median, IQR) hours | 28 (24 - 48) |
| Catheter last flushed (Min - Max) hours | 1 - 24 |
| Catheter last flushed (Median, IQR) hours | 4 (3 - 6) |
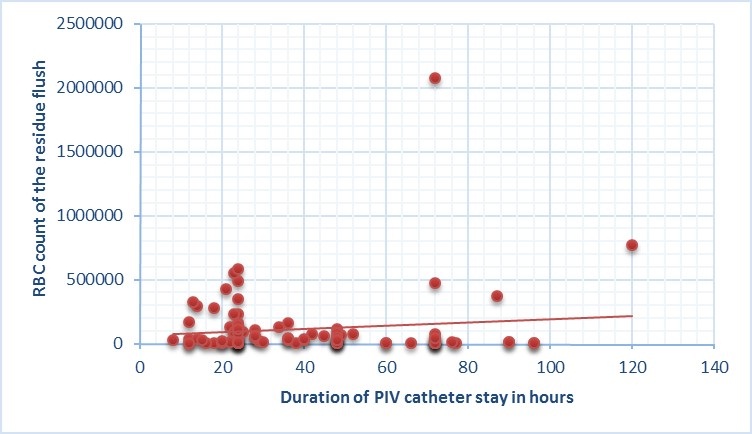
Before microscopic analysis, naked eye observation revealed 84 % of the residual flush to be blood tinged and 15% showed blood clot. On microscopic examination, all 100% of the samples revealed RBCs. [Table/Fig-3] depicts the RBC count of residual flush in our study. There was a weak correlation between the duration of PIV catheter stay in situ and the RBC counts. The Pearson product moment correlation ‘r’ was 0.12 [Table/Fig-4].
Visible blood and RBCs in the catheter Hub.
| Parameter | Number (%) |
|---|
| Blood tinge (n,%) | 78 (83.8) |
| Blood Clot (n,%) | 14 (15) |
| Microscopic RBC seen (n,%) | 94 (100) |
| RBC count (Min - Max) /cumm | 170 - 2080000 |
| RBC count (Median, IQR) / cumm | 36960 (10000 - 113920) |
Min = Minimum, Max = Maximum, RBC = Red Blood Cells, IQR = Inter quartile range
Correlation between the duration of catheter in situ and RBC count from the Hub.
Discussion
Our study found blood contamination of the PIV hub in all (100%) of the PIV catheters. Catheter related infection has been a long standing challenge in neonatal intensive care units. The standard practice of PIV catheter care in our unit is to fasten a Bionector to the hub. The Bionector is cleaned with alcohol swab before each use, medications are administered and this is followed by 1ml normal saline push over 30 seconds. The Bionector is cleaned again with the alcohol swab and left in place. As soon as the indication for having PIV catheter ceases, catheter is removed and discarded as per the hospital policy.
The major determinants of catheter related infection are breech in the sterility during insertion and handling of vascular catheters. The safety devices such as Bionector, which are attached to the vascular catheter, provide a closed system. This prevents the back flow of blood and entry of organisms from outside surroundings when in closed position.
Blood from the vein can flow back into the vascular catheter hub during administration of medications. This blood is flushed back into the vein after administering medications. If there is residual blood left over in the hub, this can facilitate the growth of bacteria. A tiny inoculum of the surrounding commensals can grow in this good culture medium. The consequence of this can range from mild thrombophlebitis to fatal sepsis.
There are several studies in the neonatal literature looking into the magnitude of catheter related infections [5,6] and also different flushing techniques to reduce catheter hub contamination [7,8]. Most of them have been conducted on central venous catheters. There is no clear data on similar issues with peripheral venous catheters. The experimental in vitro studies have used external indicators such as proteins or bacterial inoculum to assess the efficacy of different flushing techniques to reduce the catheter hub contamination [9]. In this study, we attempted to quantify the magnitude of hub contamination in peripheral venous catheter in real life scenario using patient’s own RBCs as the indicator of contamination. Our study has shown contamination of the catheter hub in all the cases studied indicating need for impact assessment. Duration of stay did not have influence on the hub contamination.
The concentration of RBCs in the hub after flush noted in our study varied from as low as 170/cumm to as high as 20,80,000/cumm. This can act as a good culture media for the bacteria to grow if inoculated even in small numbers. Our findings warrant revisiting the current flushing technique and exploration of new techniques in real life scenario to minimize PIV catheter hub contamination.
Limitation
This study did not look into how many of the neonates from whom the catheter was obtained did have either local or systemic infection.
Conclusion
Blood contamination of the small bore peripheral intravenous catheter is universal in spite of regular continuous flushing in neonatal population. We found blood contamination in all the peripheral venous catheters. Majority of them were visible to naked eye. There was no correlation between the duration of stay and magnitude of blood contamination. We are planning to check for microbial contamination in such contaminated catheters in our future study.
Min = Minimum, Max = Maximum, RBC = Red Blood Cells, IQR = Inter quartile range
[1]. Buchman AL, Spapperi J, Leopold P, A new central venous catheter cap: decreased microbial growth and risk for catheter-related bloodstream infectionJ Vasc Access 2009 10(1):11-21. [Google Scholar]
[2]. Salzman MB, Isenberg HD, Rubin LG, Use of disinfectants to reduce microbial contamination of hubs of vascular cathetersJ Clin Microbiol 1993 31(3):475-79. [Google Scholar]
[3]. Capell S, Liñares J, Sitges-Serra A, Catheter sepsis due to coagulase-negative staphylococci in patients on total parenteral nutritionEur J Clin Microbiol 1986 5(1):40-42. [Google Scholar]
[4]. Begala JE, Maher K, Cherry JD, Risk of infection associated with the use of Broviac and Hickman cathetersAm J Infect Control 1982 10(1):17-23. [Google Scholar]
[5]. http://www.cdc.gov/HAI/settings/outpatient/basic-infection-control-prevention-plan-2011/central-venous-catheters.html. Accessed on 3rd April 2016 [Google Scholar]
[6]. Fletcher S, Catheter-related blood stream infectionContin Educ Anaesth Crit Care Pain 2005 5:49-51. [Google Scholar]
[7]. Guiffant G, Durussel JJ, Merckx J, Flaud P, Vigier JP, Mousset P, Flushing of intravascular access devices (IVADs) – efficacy of pulsed and continuous infusionsJ Vasc Access 2012 13(1):75-77. [Google Scholar]
[8]. Royon L, Durussel JJ, Merckx J, Flaud P, Vigier JP, Guiffant G, The fouling and cleaning of venous catheters: a possible optimization of the process using intermittent flushingChem Eng Res Des 2012 90(6):803-07. [Google Scholar]
[9]. Ferroni A, Gaudin F, Guiffant G, Flaud P, Durussel JJ, Descamps P, Pulsative flushing as a strategy to prevent bacterial colonization of vascular access devicesMedical Devices 2014 7:379-83. [Google Scholar]