Life expectancy of patients with MPN is strongly compromised by the haemostatic complications, like vascular thrombosis and haemorrhages [1]. These patients present with arterial or venous thrombosis. Arterial thrombosis accounts for 60%-70% of events related to myeloproliferative neoplasms and it includes ischemic stroke, acute myocardial infarction, and peripheral arterial occlusion [2].
These patients can also present with deep venous thrombosis of the lower extremities, pulmonary embolism, intra-abdominal (hepatic, portal and mesenteric) and cerebral vein thrombosis. In PV patients, venous thrombosis is relatively common and constitutes approximately one-third of total events. Many a times splanchnic and cerebral vein thrombosis is the presenting feature in patients of MPN. Also, the prevalence of splanchnic and cerebral vein thrombosis is higher in these patients [2]. Thrombotic complications not only involve large blood vessels but also involve microvasculature causing digital ischemia, pregangrenous changes, erythromelalgia, dizziness, confusion, headache, seizures and visual disturbances [3].
Patients of MPN are in a hypercoagulable state even in the absence of frank thrombotic manifestations. This is proven by the increased levels of plasma biomarkers of haemostatic system activation [4–6].
This study was undertaken to assess the haemostatic defects and platelet functions in patients of MPN.
Materials and Methods
This was a prospective study of one year duration from (August 2014 to July 2015) conducted in a tertiary care centre of North India. Fifty patients of MPN diagnosed after peripheral smear and bone marrow evaluation were included in this study. Patients taking vitamin K, antiplatelet/antithrombotic drugs, antimyeloproliferative drugs, patients with liver disease, high fever, patients on OCPs and pregnant patients were excluded from this study. Apart from this, 30 age and sex matched normal healthy individuals who were not receiving drugs interfering with platelet functions or coagulation were selected as controls.
All the patients underwent screening investigations like CBC, peripheral smear evaluation, BT, PT, aPTT, Protein C and S measurement (clot based assay using reagents from Tulip Diagnostics, India) and aggregation studies with ADP (5μM) (Optical Aggregometry with AGGRO/LINK 8 software and CHRONOLOG 700 aggregometer).
Continuous data were summarized as Mean±SD (standard deviation) while discrete (categorical), in number and percentage (%). Continuous groups were compared by Student’s t-test. Categorical groups were compared by Chi-square (χ2) test. A two-tailed p<0.05 was considered statistically significant. Analyses were performed on SPSS software (Windows version 17.0). Ethical clearance was obtained for this study.
Results
A total of 50 cases were included in present study, out of which 42 were of CML, 2 each of CNL, ET, PMF and one each of PV and MPN-NOS. [Table/Fig-1] is showing the age distribution of cases. In the present study, out of 50 patients, 16 (32.0%) were females and 34 (68%) were males. Both patients of ET and single patient of PV were males. Of the total, 69% patients of CML were males, while sex distribution in CNL and PMF was 1:1. Thirty age and sex matched control were included.
Number of patents in each age group.
| Age Group (years) | CML-(CP+BP) | CNL | ET | MPN-NOS | PMF | PV |
|---|
| Upto 10 | 2 | - | - | - | - | - |
| 11-20 | 1 | - | - | - | - | - |
| 21-30 | 6 | - | - | - | - | - |
| 31-40 | 20 | - | 1 | 1 | - | - |
| 41-50 | 10 | 1 | 1 | - | 1 | - |
| >50 | 3 | 1 | - | - | 1 | 1 |
| Total | 42 | 2 | 2 | 1 | 2 | 1 |
Out of 50 patients, only 8 patients had haemorrhagic manifestations. None of patients presented with clinical frank thrombosis.
Haemorrhagic manifestations noted were epistaxis, melena, menorrhagia, gum bleeding, haematoma and petechial rashes. One patient of CML presented with subdural haematoma and one ET patient presented with petechial rashes mostly on lower limbs and forearms.
Difference in values for all the coagulation variables including platelet aggregation, in controls and cases was found to be statistically significant. Values of Maximal Aggregation with ADP 5μM, Protein C and Protein S in cases were found to be lower than that of controls while values of BT, PT, aPTT, MPV and PDW was found to be higher in cases as compared to controls [Table/Fig-2].
Comparison of various coagulation parameters between controls (n=30) and cases (n=50).
| Variables | Control (n=30) | Cases (n=50) | Statistical Significance (Student t-test) |
|---|
| Mean±SD. | Mean±SD | ‘t’ | ‘p’ |
|---|
| Bleeding time (BT) (in minutes) | 2.73±0.64 | 4.74±2.90 | -3.724 | <0.001 |
| Activated partial thromboplastin time (aPTT) (in seconds) | 28.30±5.59 | 32.00±5.23 | -2.984 | 0.004 |
| Prothrombin time (PT) (in seconds) | 12.50±1.36 | 13.41±1.67 | -2.522 | 0.014 |
| Maximal aggregation (%) | 78.97±5.31 | 52.57±23.40 | 6.072 | <0.001 |
| Protein C (% activity) | 96.03±10.20 | 60.67±22.88 | 7.987 | <0.001 |
| Protein S (% activity) | 104.2±12.72 | 58.40±25.88 | 9.044 | <0.001 |
| Mean platelet volume MPV(fl) | 8.73±1.10 | 10.45±1.67 | -5.008 | <0.001 |
| Platelet distribution width PDW (%) | 17.23±2.40 | 29.48±20.31 | -3.282 | 0.002 |
Maximal platelet aggregation, Protein C and S were found to be significantly lower in our patients of MPN as compared to controls (p<0.001). Mean platelet volume (MPV) was found to be significantly higher in our MPN cases as compare to controls (p<0.001).
Values of PDW showed that patients of myeloproliferative disorders have higher variation in size of their platelets. This variation was found to be more in ET patients; such variations represented as high values of standard deviation in our patients of MPN. Further differences in mean PDW of cases and controls were highly significant with p-value <0.01.
Out of 50 cases, only 8 patients had haemorrhagic manifestations. Difference between BT of haemorrhagic and non-haemorrhagic cases was statistically significant (p<0.0001) [Table/Fig-3]. Haemorrhagic manifestations seen in seven patients of CML included episodes of epistaxis, melena, menorrhagia, gum bleeding, haematoma and petechial rashes. One patient of CML presented with chronic subdural haemorrhage [Table/Fig-4].
Comparison between non-haemorrhagic and haemorrhagic cases.
| Variables | Non-Haemorrhagic (n=42) | Haemorrhagic (n=8) | Statistical Significance |
|---|
| Mean ± SD | Mean ± SD | t | ‘p’ |
|---|
| Bleeding time (minute) | 3.71 | 1.73 | 10.12 | 1.42 | 9.8420 | <0.0001 |
| aPTT (second) | 31.57 | 5.06 | 34.25 | 5.90 | 1.3384 | 0.1871 |
| PT (second) | 13.39 | 1.69 | 13.50 | 1.69 | 0.1687 | 0.8667 |
| Platelet count (cumm) | 290381 | 142181 | 370000 | 253068 | 1.2653 | 0.2119 |
| MPV (fl) | 10.49 | 1.76 | 10.23 | 1.07 | 0.4019 | 0.6896 |
| PDW (%) | 27.93 | 18.25 | 37.60 | 29.09 | 1.2412 | 0.2206 |
| Maximal aggregation (ADP 5μM) (%) | 52.21 | 23.99 | 54.45 | 21.39 | 0.2458 | 0.8069 |
| Protein C (% activity) | 61.70 | 23.24 | 55.25 | 21.43 | 0.7274 | 0.4705 |
| Protein S (% activity) | 60.06 | 25.63 | 49.66 | 27.15 | 1.0426 | 0.3023 |
CT scan - chronic subdural hemorrhage in fronto-parietal region.
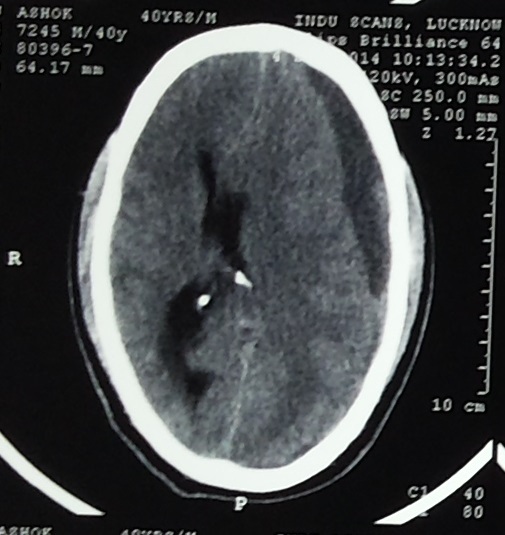
Other variables showed only small differences between haemorrhagic and non-haemorrhagic MPN patients. Patients of CML-CP, ET, PV, PMF, MPN-NOS had reduced maximal aggregation with ADP (5μM) [Table/Fig-5,6].
Subtypes of MPN and maximal platelet aggregation (ADP 5μM).
| MPN-subtype | Number of cases | Maximal percentage aggregation (%) |
|---|
| Mean | Std. dev. (± SD) | Minimum | Maximum |
|---|
| CML-BP | 1 | 79.00 | - | 79 | 79 |
| CML-CP | 41 | 55.02 | 22.81 | 12 | 105 |
| CNL | 2 | 67.00 | 2.83 | 65 | 69 |
| ET | 2 | 26.30 | 13.43 | 17 | 36 |
| PV | 1 | 44.00 | - | 44 | 44 |
| PMF | 2 | 20.00 | 4.24 | 17 | 23 |
| MPN-NOS | 1 | 23.00 | - | 23 | 23 |
Reduced platelet aggregation with ADP 5 μM.
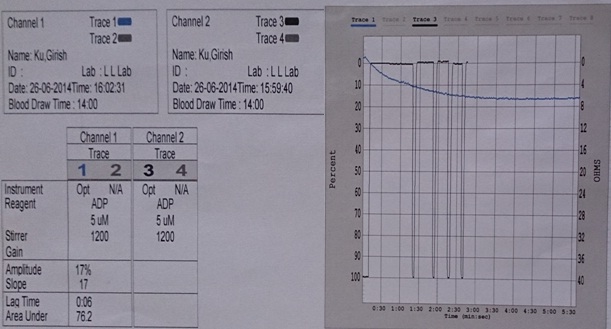
Discussion
Thrombohaemorrhagic complications in MPN are common morbid conditions. In present study there was an occult prothrombotic state, but no patient presented with frank thrombosis while eight out of fifty patients had haemorrhagic manifestations ranging from subdural haematoma to pin point petechial haemorrhages.
In present study, the only patient of PV, presented with symptoms suggestive of microcirculatory disturbances (headache, dizziness, pruritus) but no frank thrombosis was seen.
One of ET patients presented with petechial rashes on the lower limbs; suggestive of qualitative platelet defect or defective vascular endothelial cells. Patients of PMF, CNL and MPN–NOS didn’t show any thrombotic or haemorrhagic manifestations.
Another inference which can be made is that patients with normal or even elevated platelet count can present with raised BT and haemorrhagic manifestations; this finding gives us an insight of defective platelet functions in MPN as stated by previous workers [7–14]. Pathogenesis behind such findings includes increased expression of P-selectin and tissue factors on platelets in MPN patients which may lead to exhaustion of functional ability of platelets to aggregate. Another paradox is that very high number of platelets as seen in ET patients (8,73,000 and 5,60,000/mm3) may consume all available large circulatory vWF multimers and results in increased bleeding time and haemorrhagic manifestations [15].
In present study, there were significantly reduced levels of maximal platelet aggregation with ADP (5μM) (52.57±23.40%) as compared to healthy controls (78.97±5.31%) with p<0.001 [Table/Fig-2]. Such results have been found in many studies as mentioned in [Table/Fig-7] [16–20]. All of these studies emphasize on the loss of secondary wave of aggregation and reduced maximal platelet aggregation.
Comparison of platelet aggregation (%) in different studies and in present study [16–20].
| Author | Platelet aggregation (abnormal cases / total cases) | Patient Population |
|---|
| ADP | Collagen | Epinephrine |
|---|
| Cesar JM (2005) [16] | 6/55 | 11% | 21/55 | 38% | 32/55 | 58% | ET only |
| Avram S (2001) [17] | 19/76 | 25% | - | - | - | - | All MPN |
| Waddell CC (1981) [18] | 9/18 | 50% | 7/18 | 38.9% | 11/18 | 61.1% | All MPN |
| Pareti FI (1982) [19] | 19/52 | 36.5% | 9/52 | 17.3% | 15/52 | 28.8% | All MPN |
| Russell NH (1981) [20] | 9/17 | 52.9% | 10/17 | 58.8% | - | - | All MPN |
| Present study | 40/50 | 80% | - | - | - | - | All MPN |
These abnormalities can be justified by the defective arachidonic acid metabolism, reduced activation of platelets by agonists, acquired storage pool disease, decreased number of α2-adrenergic receptors, altered expression of specific platelet membrane Glycoproteins (GP) such as GPIIb/IIIa, GPIb/IX or increased number of GPIV molecules and increased expression of receptors for the Fc component of IgG, acquired von Willebrand’s disease, a reduction of platelet procoagulant activity and an acquired form of Bernard-Soulier syndrome as reported in these disorders by other researchers [7–23].
In present study, activity of Protein C was found to be reduced very significantly [Tables/Fig-2,3] in MPN cases (60.67±22.88%) as compared to the mean activity in controls (96.03±10.20%) with p<0.001. Similar findings are seen in studies done by other workers and they found an acquired activated Protein C resistance in MPN cases [Table/Fig-8] [24–26].
Comparison of Protein C and S activity in different studies [24–26].
| Author | Number of patients with deficiencies of PC and PS | Percentage |
|---|
| Bucalossi A (1996) [24] | 22/81 | 27.16% |
| Amitrano L (2003) [25] |
| Protein C | 7/61 | 11.4% |
| Protein S | 7/79 | 8.8% |
| Hoekstra J (2011) [26] | 4/14 | 28.6% |
| Present Study |
| Protein C | 34/50 | 68% |
| Protein S | 30/50 | 60% |
Low levels of Protein C indicate the presence of “Prothrombotic State” in MPN patients even in the absence of frank thrombosis.
Protein S activity was also found to be reduced in MPN cases [Table/Fig-2] (58.40±25.88%) in the present study as compared to activity of Protein S in healthy controls (104.20±12.72%).
Such kind of differences have also been seen by other researchers. These results can be justified by the researchers which indicate that proteases from platelets and leukocytes are able to cleave Protein S in plasma [27]. Separate studies done by Marchetti M et al., and Falanga A et al., showed that increased neutrophil elastase levels in MPN patients degrade Protein S. Studies also showed that factor V also undergo similar degradation by elastases [28–30].
Falang A et al., suggested that increased blood coagulation in MPN patients is multifactorial and it involves abnormalities in platelets, erythrocytes, leucocytes and endothelial cells. Sekhar M et al., found splenic vein thrombosis to be one of the most common presentations of MPN. However, in our studies no such cases were found. Martinelli I et al., found that MPN patients are at increased risk of cerebral vein thrombosis [31–33].
MPV of MPN cases (10.45±1.67fl) was found to be statistically higher in comparison with that of controls (8.73±1.10) having p <0.001 [Table/Fig-2]. These higher values of MPV are suggestive of presence of large sized platelets in MPN patients.
PDW of MPN cases (29.48±20.31%) was compared with that of controls (17.23±2.40%) and was found to be significantly higher in MPN cases (p<0.01) [Table/Fig-2].
Higher value of PDW in MPN cases is an indicator of presence of variable sized platelets in MPN patients as seen in various studies [34,35].
In present study, MPV was correlated with maximal platelet aggregation (categorized as <70% and ≥70%); a statistically significant correlation was found (p<0.05) as patients with higher MPV had reduced maximal platelet aggregation.
Limitation
This study was of short duration and effect of MPN treatment on coagulation profile was not studied.
Conclusion
Thrombohaemorrhagic complications significantly affect the morbidity and mortality of MPN patients. These are multifactorial events that include the key role of platelets with their in vivo activation and role of vascular endothelial cells, leukocytes, and reduced activity of Protein C and S. Also, these patients are in a continuous hypercoagulable state even in the absence of frank thrombosis. Hence, patients of MPN, should be monitored and counselled appropriately keeping these complications in view.
Abbreviations
MPN: Myeloproliferative Neoplasm
BT: Bleeding Time
PT: Prothrombin Time
aPTT: Activated Partial Thromboplastin Time
ADP: Adenosine Diphosphate
MPV: Mean Platelet Value
CML-CP: Chronic Myeloid Leukamia in Chronic Phase
ET: Essential Thrombocythemia
PV: Polycythemia Vera
PMF: Primary Myelofibrosis
MPN-NOS: Myeloproliferative Neoplasm-Not Otherwise Specified
OCPs: Oral Contraceptive Pills
MPV: Mean Platelet Volume
CBS: Complete Blood Count
CNL: Chronic Neutrophilic Leukamia
PDW: Platelet Distribution Width