Primary Pulmonary Primitive Neuro-Ectodermal Tumour (PNET) in an Eight-Year-Old Girl - A Rare Case
Radhika Narayan1, J Sreedevi2, Farah Rana3, Minakshi Mishra4, Rajesh Mohanty5
1 Specialist, Department of Pathology, Tata Main Hospital, Jamshedpur, Jharkhand, India.
2 Senior Registrar, Department of Pathology, Tata Main Hospital, Jamshedpur, Jharkhand, India.
3 Associate Specialist, Department of Pathology, Tata Main Hospital, Jamshedpur, Jharkhand, India.
4 Senior Specialist and HOD, Department of Pathology, Tata Main Hospital, Jamshedpur, Jharkhand, India.
5 Specialist, Department of Pathology, Tata Main Hospital, Jamshedpur, Jharkhand, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Radhika Narayan, Department of Pathology, Tata Main Hospital, Jamshedpur-831001, Jharkhand, India.
E-mail: drrnarayan@tatasteel.com
Primitive Neuro-Ectodermal-Tumours (PNET) and Ewing’s sarcoma are part of the spectrum of Ewing’s Family of Tumours (EFT) and show varying degrees of neuroectodermal differentiation. Both these tumours share similar histological and genetic features. PNETs arising primarily in the lungs without pleural or chest wall involvement are extremely rare. We report a case of pulmonary PNET in an eight-year-old girl. To the best of our knowledge, this is the youngest case of primary pulmonary PNET to be reported in paediatric age group in the Indian literature.
CD 99, Immunohistochemistry, Ewing’s sarcoma
Case Report
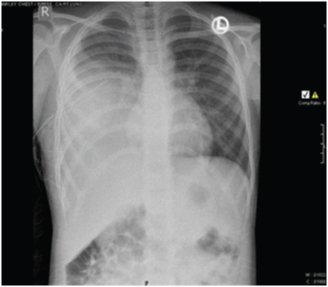
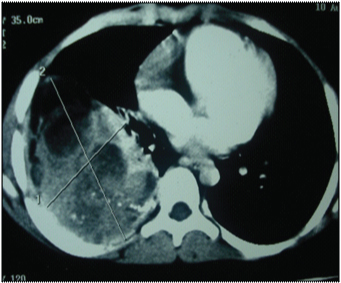
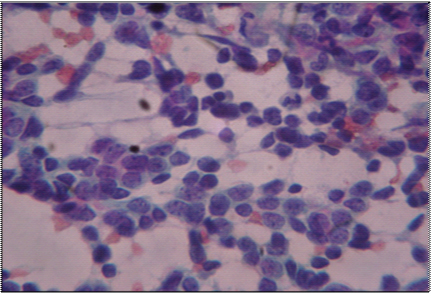
An eight-year-old girl presented to our hospital with the chief complaints of having low grade fever, off and on since 2 months with evening rise of temperature which was not associated with chills, rigor or rashes. She was the younger of two siblings and there was no history of malignancy in either of them. There was no pallor, icterus, clubbing, cyanosis or any lymphadenopathy. On examination of the respiratory system, chest was bilaterally symmetrical, trachea was midline. Chest expansion was diminished in right lower part. Tactile and vocal fremitus was diminished over right infra mammary, infra axillary and infrascapular region with dullness and vesicular breath sounds over same. Other systemic examination was normal. Her routine haematological and biochemical investigations were normal. Pulmonary function test showed a mild obstructive ventilatory defect. Her Chest X-ray showed right sided pleural effusion with mass in the right lung [Table/Fig-1]. A CT scan of the chest showed a large lobulated soft tissue mass measuring approximately 90x60 mm in the right lung with multiple irregular calcified foci within [Table/Fig-2]. The mass was overlying the right posterolateral ribs, abutting the vertebra and displacing the hemithorax superomedially. Postero-medially, the mass was abutting the vertebra, though no obvious bony destruction was noted. Rib destruction was seen but no obvious invasion was noted. There was minimal right sided pleural effusion. Bilateral lung fields, trachea, bronchi were normal and there was no lymphadenopathy. Based on the above findings, a clinical impression of right lung mass with right pleural effusion was made. Transthoracic Ultrasonography (USG) guided Fine Needle Aspiration Cytology (FNAC) of the mass was done which showed groups of round to oval tumour cells with stippled nuclear chromatin, inconspicuous nucleoli and scanty cytoplasm [Table/Fig-3]. In focal areas attempted rosette formation was also seen. A diagnosis of malignant small round cell tumour was made.
Chest X-ray showing mass in the right lung with right pleural effusion.
Contrast enhanced CT of chest showing a large lobulated soft tissue mass (9.0x6.0 cm) in the right lung overlying the right posterolateral ribs, with multiple irregular calcific foci within.
(PAP stain x 400)- Ultrasound guided FNAC of the right lung mass showing groups of round to tumour cells with hyperchromatic nuclei, scanty cytoplasm and occasional nucleoli.
A whole body bone scan was done which showed abnormal uptake in shaft of 6th to 9th ribs. However there was no evidence of metastasis.
Thoracotomy and open biopsy of right lung mass was done. In a left lateral position, incision was given over right 8th intercostal space, thorax was opened, haemorrhagic fluid drained and was sent for cytology. Punch biopsy of tumour tissue was taken and sent for histopathological examination. Intercostal tube drainage was put and 50ml of serosanguinous discharge was drained. Two units of packed cells were transfused, and the post-operative recovery was uneventful.
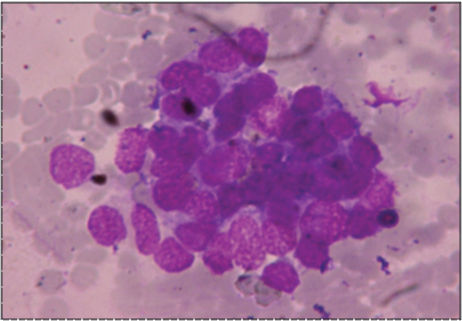
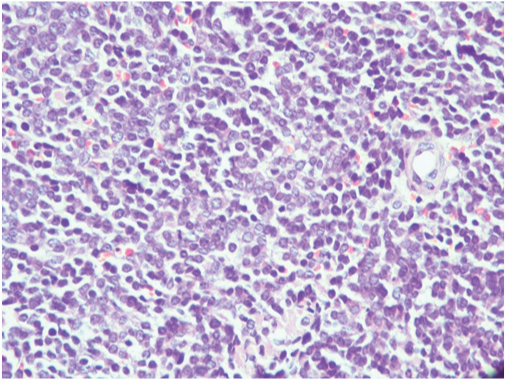
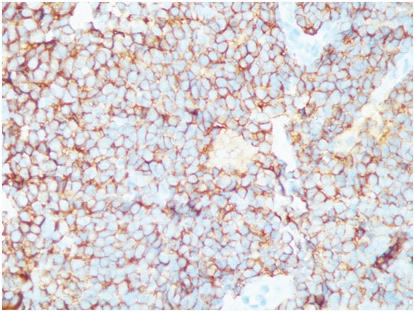
Cytological examination of the haemorrhagic fluid obtained intra-operatively showed mildly pleomorphic round to oval cells with hyperchromatic nuclei, arranged in clusters and sheets. Above findings were suggestive of undifferentiated small cell carcinoma of lung [Table/Fig-4]. Biopsy of the lung mass showed highly cellular tumour arranged in sheets and nests with scanty stroma with areas of haemorrhage and necrosis. The cells had round to oval nuclei with vesicular chromatin, inconspicuous nucleoli and scanty cytoplasm. Increased mitotic activity was seen [Table/Fig-5,6]. Immunohistochemistry was done at a higher center and the tumour cells showed cytoplasmic membrane staining for CD99 (MIC2) consistent with PNET [Table/Fig-7]. Cytogenetic analysis was not done. Work up to exclude primary tumour elsewhere was negative. A final diagnosis of primary pulmonary PNET was given. The patient was treated at a higher center with surgery and chemotherapy and continues to be well and on regular follow-up for the last 5 years.
(MGG x 400)- cytology of haemorrhagic pleural fluid obtained pre operatively – showed clumps of tumour cells with hyperchromatic nuclei.
Biopsy of right lung mass showing highly cellular tumour in nests and sheets separated by scanty stroma (H&E x 200).
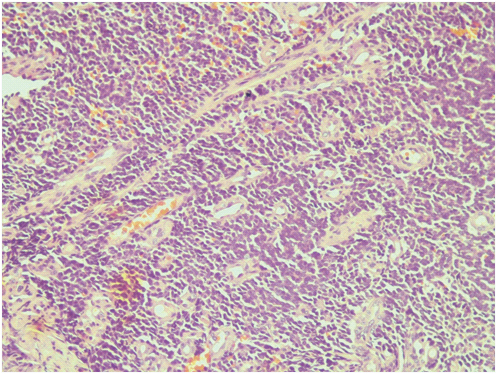
High power showing tumour cells with round to oval nuclei, vesicular chromatin, inconspicuous nucleoli (H&E x 40x).
Tumour cells show strong membrane positivity for MIC 2 (CD 99,40x).
Discussion
Ewing’s Sarcoma/PNET are small round cell tumours showing a varying degree of neuroectodermal differentiation. Both Ewing’s sarcoma and PNET show similar translocations and are considered to be the ends of a histological spectrum of “Ewing’s Family of Tumours” (EFT) [1]. They occur most frequently in the bones and soft tissues of children or young adults. Bones commonly involved are femur, humerus and the pelvic bones. Soft tissue lesions arise more frequently in the deep tissues of the paravertebral or thoracic regions, lower extremities and pelvis [2].
Primary pulmonary ES/PNETs are extremely rare and metastasis from elsewhere has to be ruled out before rendering a diagnosis of primary ES/PNET. Though lung metastasis by this tumour is common, primary intrapulmonary PNET without involvement of parietal pleura or thoracic wall is very rare. Most patients with pulmonary PNET described earlier had an age range of 15-67 years with mean age of 33 years [3]. Our patient presented with nonspecific symptoms of only fever without specific symptoms of lung disease like majority of the reported cases [4]. Most cases have a lung mass on chest X-ray with or without calcification.
The diagnosis is based on a combination of light microscopic, cytogenetic and immunohistochemistry features. Light microscopic features show monomorphic small round tumour cells which are glycogen positive. On immunohistochemistry, these tumours are positive for MIC2 (CD 99), NSE, S-100 and negative for LCA, Epithelial membrane antigen, cytokeratin and desmin [3]. Demonstration of the t(11:22)(q24:q12) chromosomal translocation (EWS-FLI1) gene rearrangement is highly specific for ES/PNET and is demonstrated in more than 90% of cases.
The differential diagnosis should include other metastatic or primary round cell tumours like neuroblastoma, lymphoblastic lymphoma and rhabdomyosarcoma. Immunohistochemical expression of MIC2 gene product (CD99) is useful in separating this case from other small cell tumours as was done in this case. Two other primary lung malignancies in infancy and childhood that need to be considered in differential diagnosis are Pleuropulmonary blastoma and carcinoid tumours [5]. Pleuropulmonary blastoma is a rare aggressive malignant tumour of infancy and early childhood that is pulmonary and/or lung based. It is the pulmonary analog of other childhood tumours including Wilm’s tumour, neuroblastoma, hepatoblastoma [6]. The tumour mostly affects children in the age range of one month to twelve years. Most patients present with respiratory symptoms like cough, dyspnea, chest pain and respiratory distress with or without pneumothorax. It shows a mixture of primitive blastematous and sarcomatous elements, epithelial component is entirely lacking or present in the form of benign looking presumably entrapped epithelium. Some of these tumours may be entirely cystic [6]. On IHC these tumour cells are positive for CD 45, CD117 and weakly positive for CD 99 and negative for synaptophysin, chromogranin.
Shet N et al., have reviewed the literature on the previously reported 10 cases of primary extraosseous pulmonary Ewing’s sarcoma of the lung [7]. Only one patient was an eight-year-old boy, while the remaining nine patients were more than 10 years of age. To the best of our knowledge our case report in this eight-year-old girl is the youngest age in which this very uncommon tumour has been reported in Indian literature.
Conclusion
Though very rare, pulmonary PNET should be considered in the differential diagnosis of any primary pulmonary tumour specially in children or young adults, especially if a primitive small round tumour is encountered in cytology or histology.
[1]. Desai SS, Jambhekar NA, Pathology of Ewing’s Sarcoma/PNET:Currentopinion and emerging conceptsIndian J of Orthop 2010 44:363-68. [Google Scholar]
[2]. Gaude GS, Patil P, Malur P, Annurshetru S, Primitive neuro-ectodermal tumour of the lungJ Thorac Dis 2015 7:682-85. [Google Scholar]
[3]. Amita RN, Sandhyamani S, Balasubramoniam KR, Primary primitive neuroectodermal tumour of lung: A case reportIndian J Pathol Micrbiol 2013 56:479-80. [Google Scholar]
[4]. Suárez Antelo J, Rodríguez García C, Montero Martínez C, Verea Hernando H, Pulmonary Ewing sarcoma/primitive neuroectodermal tumour: A case report and a review of the literatureArch Bronconeumol 2010 46:44-46. [Google Scholar]
[5]. Yu DC, Grabowsi MJ, Kozakewich HP, Perez-Atayde Voss SD, Shamberger RC, Primary lung tumours in children and adoloscents: a 90 year experienceJ Pediatr Surg 2010 45(6):1090-95. [Google Scholar]
[6]. Yeh YA, Edelman MC, Lung: PleuropulmonaryblastomaAtlas Genet Cytogenetoncol Haematol 2011 15(4):374-77. [Google Scholar]
[7]. Shet N, Stanescu L, Deutsch G, Primary extraosseous Ewing sarcoma of the lung: Case report and literature reviewRadiology Case Reports. (Online) 2013 8:832 [Google Scholar]