Epilepsy is one of the most common neurological disorders worldwide, affecting 70 million persons, roughly one-sixth of whom reside in India [1]. Temporal Lobe Epilepsy (TLE), the most common type of epilepsy in humans [2], often presents in childhood or adolescence [3] and therefore affects critical stages in cerebral cortical development of learning and memory [4]. The kainate model of TLE takes advantage of the ability to create lesions and kindle seizures in the deep temporal limbic cortex of the rat [5]. Seizures generated by the hippocampus and amygdala, key hubs in the brain’s learning and memory networks, are known to occur in humans affected by TLE [6]. Such seizures stimulate neurogenesis in the hippocampus [7], one of the key sites of neurogenesis in the mammalian brain [8]. Neurogenesis is thought to be an adaptation to the recurrent seizures associated with TLE [9]. Neurogenesis induced by antiepileptic drugs and therapeutic treatments may also ameliorate the adverse effects of seizure activity on learning and memory functions by a cannabinoid receptor mechanism [10,11]. However, treatment with valproic acid is shown to inhibit seizure-induced neurogenesis by an epigenetic mechanisms involving deacetylation of histones [12]. Swimming exercise (Ex)is shown to stimulate neurogenesis in laboratory animals [13], partly by an antioxidant mechanism [14]. A previous study from our laboratory has shown that daily swimming Ex for 30 days stimulates neurogenesis in rats and appears to be effective at ameliorating the adverse effects of kainate-induced seizures on neurogenesis, whether the Ex regimen is initiated immediately or after a two-month delay, postictally [15]. The present investigation is an expansion of this prior study, intended to determine whether a similar Ex regimen in rats with kainate-induced seizures ameliorates the learning and memory deficits associates with such seizures, thereby demonstrating behavioural changes that may be correlated with Ex induced neurogenesis.
The present study was designed to test the hypothesis that swimming Ex, begun in either the immediate postictal period or after a significant delay following KA-induced seizures, ameliorates epilepsy-related deficits in learning and memory.
Materials and Methods
This research was approved by the Ethics Committee, Manipal University, Manipal and was conducted in 2012.
Animals
A total of 60 male Wistar rats (four-month-old) were used. All the cages were maintained in 12hr light and 12hr dark cycle in well-ventilated rooms within the Manipal University Animal House. All rats were fed ad libitum with a balanced diet containing 21.96% crude oil, 3.10% crude fiber, 7.37% ash, 1.38% sand silica [15].
Experimental Design
A total of 60 rats were randomly assigned to groups of 12 animals, as follows: (1) normal control (NC); (2) normal control + exposure to exercise (NC+EX); (3) sham control + exposure to exercise (SC+EX); (4) kainic acid lesioned (LO); and (5) kainic acid lesioned + exposure to exercise (L+EX). Rats in the normal control group remained undisturbed in the home cage. Rats in the NC+EX were subjected to swimming Ex for 15 min/day. Rats in the sham control group were subjected to sham surgery. The sham surgery consisted of positioning the rats fixed in a stereotaxic apparatus (Digital Lab Stereotaxic Instrument, Braintree Scientific, Inc., Braintree, MA 02185). Burr holes were drilled in the skull using appropriate coordinates. A Hamilton syringe was lowered into the lateral ventricles bilaterally and removed in the sham-operated animals. The scalp wounds were sutured, animals were replaced back in their home cage and were subjected to Ex. Rats in the lesion only group were administered kainic acid (KA) bilaterally into the lateral ventricles using a Hamilton syringe [15].
Experimental Procedures
An excitotoxic lesion was created in the hippocampus by injecting KA into the lateral ventricles [15]. Rats were first anaesthetized with a cocktail of ketamine (50mg/ml), xylazine (4.5mg/ml) and acepromazine (0.4mg/ml) at a dose of 0.70ml/kg body weight and were fixed in the stereotaxic apparatus in such a way that the incisor bar was 3.7mm below the interaural plane. The skull was exposed and a burr hole was drilled using the following coordinates on the right and left sides: Anteroposterior 3.7mm behind the bregma 4.1mm lateral to the midline [15]. A Hamilton syringe needle filled with (KA) (0.5μg/μl) was lowered by 4.5mm to reach the lateral ventricle and 1.0μl of KA was injected slowly over a period of 20 min. The needle was withdrawn, skin was sutured and the animals were kept warm until recovery from anaesthesia. Lesioned animals were housed individually. Sham surgery was performed to rule out the effect of surgical injury. Here rats were anaesthetized, fixed in the stereotaxic apparatus and burr hole was drilled as described above. A Hamilton syringe needle was lowered and held in position for 20min and then withdrawn. The skin was sutured and the animals were returned to their home cages [15]. This method is shown to result in lesioning of the hippocampus, amygdala and motor cortex [15].
Exercise Intervention
A total of 36 rats were subjected to swimming Ex in a water tank (25°C, 1.5 m diameter) for 15 min daily for 30 days. This swimming Ex protocol is shown to increase neurogenesis in hippocampus, amygdala and motor cortex [15].
Behavioural Tests
The animals in each group were subjected to the following behavioural testing: T-maze tests of spatial learning and passive-avoidance tests of short-term memory were performed on the 42nd day following grouping for the normal controls, on the 32nd day following sham surgery for the sham-operated controls, on the 32nd day following lesioning for the KA-lesioned animals and on the 32nd day for the KA-lesioned animals subjected to the immediate swimming Ex treatment (1 d after lesioning). The same T-maze and passive-avoidance tests were performed on the 62nd day for the KA-lesioned animals subjected to the delayed swimming Ex treatment (60th d, after lesioning). All testing was done at 7 pm, in accordance with the known diurnal variation of night time activity in rats [16].
T–maze Testing
Animals were subjected to left-right discrimination, a spatial memory task. This task is a test of ability to discriminate the left or right arm of a T-maze in order to acquire a food reward. Two days prior to the start of the testing, the rats were deprived of food to enhance motivation for the food reward. Subsequently, the food was restricted during trials so that the animal’s body weight was maintained at 85% of pre-test weight. Animals underwent a period of orientation, during this period they were subjected to food restriction and were placed in the start box for 60 seconds. Rats were permitted to explore the T– maze for 30 min and to eat 15 pellets (10mg each) of consistent size in each goal area. After 30 min, the rats were returned to the start box. This orientation procedure was carried out for 2 consecutive days. After the orientation period, 6 trials were conducted daily for 4 consecutive days.
Spontaneous Alternation Test
In each trial, the rat was first placed in the start box and the rat was permitted to enter into the stem of the maze and choose either of the maze arms. A rat was considered to have entered into a particular arm only when it entered that arm with all its limbs. Once the rat ate a pellet in the goal area of that arm, the animal was placed back in the start box for the next trial. The inter-trial interval was 1 min in duration. For each trial, the arm chosen by the rat was recorded. At the end of the 4 d experimental period (24) trials the total number of alternations was recorded. The percent bias was computed for each rat using the following formula:
Percent bias = {Total number of selections of most frequently selected side ÷ Total number of trials} x 100 [16].
Rewarded Alternation Test
This test was initiated one day after completion of the spontaneous alternation test. During this test, 6 trials/day were conducted for 4 consecutive days. Each trial had 2 runs, a forced and a selection run. In the forced run, the animal was forced into one of the arms by blocking the other arm and was allowed to consume the pellets in the goal area. Once the animal ate the pellets in the goal area, it was placed back in the start box for a selection run. In the selection run, the goal area of the forced arm was kept empty and pellets were placed in the goal area of the opposite arm. Both arms were accessible for the rat; a 1 min pause separated each forced and selection run and 1 min pause was separated each trial. The sequence of the forced arm was predetermined and was the same for all the rats on a given done subsequent d, the sequence was alternatively changed. During the selection run, if the rat entered the arm opposite to the forced arm, that response was recorded as “correct response.” If the rat selected the same arm that it had been forced to enter during the forced run, it was recorded as the “wrong response.” The percentage of correct responses was computed for each rat using the following formula:
Percentage of correct responses = {Total number of correct responses ÷ Total number of trials} x 100.
Passive-avoidance Task
This task consisted of the following 3 parts: i) an exploration test; ii) an aversive stimulation and learning phase (passive-avoidance acquisition); and iii) a retention test.
Exploration Test
Each rat was subjected to 3 exploration tests on the same day. The inter-trial interval was 5 min and the duration of each trial was 3 min. Each rat was kept in the center of a larger compartment facing away from the entrance to a dark, smaller compartment. The door between the two compartments was kept open. The rats were accessed to the passive avoidance apparatus for 3 min during exploration. For each trial, the total time spent in the larger compartment, the total time spent in the smaller compartment and the number of crossings from the larger to the smaller compartment, a measure of exploratory behaviour, was recorded. Each rat was used 3 times in the exploration phase of passive avoidance test and given 5 min duration between each running a day.
Aversive Stimulation and Learning Phase: Passive-Avoidance Acquisition
After the last exploration trial, each rat was forced into the smaller compartment and the sliding door between the two compartments was closed. Three strong foot shocks (50 Hz, 1.5 mA, 1second duration) were given at approximately 5-s intervals. The top cover was then opened and the rat was returned to its home cage [17].
Retention Test
The retention test was performed 24 hours after the acquisition test. The rats were kept in the center of the larger compartment facing away from the entrance to the smaller compartment, the sliding door between the two compartments was kept open and each rat was allowed to explore the apparatus for 3 min; after 3 min, each rat was returned to its home cage. This sequence was repeated 3 times with an inter-trial interval of 5 min. For each trial, the total time spent in the larger compartment, the total time spent in the smaller compartment and the number of crossings from the larger to the smaller compartment, a measure of exploratory behaviour, was recorded.
Statistical Analysis
The behavioural response variables of interest were identified as dependent variables.
Statistical significance of differences between each of the dependent variables was evaluated across the independent categorical variables (normal control, normal control + Ex, sham operated control + Ex, kainate lesion, kainate lesion + Ex groups). The assumption of normality in the distribution of least-square residuals was met by virtue of skewness and kurtosis values between -1.0 and +1.0 and statistical significance of the intergroup differences was determined by one-way analysis of variance (ANOVA) followed by Bonferroni’s post-hoc test using GraphPad Prism, version 5. Results were expressed as mean ± standard deviation (SD) and the significance level was set at p< 0.05.
Results
The swimming Ex resulted in significant increments in the percent bias [Table/Fig-1a,b], percentage of correct responses [Table/Fig-1c,d] and the number of alternations [Table/Fig-1e,f]. These increments were observed in the animals subjected to the Ex intervention one day after grouping in the normal and sham-operated controls, 1 day after KA lesioning in the experimental rats [Table/Fig-1a,c,e], 60 days after grouping in the normal and sham-operated controls and 60 days after KA lesioning in the experimental rats [Table/Fig-1b,d,f].
Effects of 30 days swimming Ex on learning T-maze task. White bars compare means ± SD showing percent bias, percentage of correct responses and number of alternations in groups of four-month-old male Wistar rats exposed to the following conditions: NC, NC+EX and SC+EX treatments. Black bars compare means ± SD of percent bias, percentage of correct responses and number of alternations in groups of four-month-old rats subjected to LO followed by L+EX. a,b): shows significant increments in the percent bias; c&d) show percentage of correct responses; e&f): number of alternations. a,c,e) 1 d post grouping for controls, 1 day postictal for lesioned rats. b,d,f)-60 d post-grouping for controls, 60 d postictal for kainate-lesioned rats. *Different than NC at p<0.05; #different than LO at p<0.001.
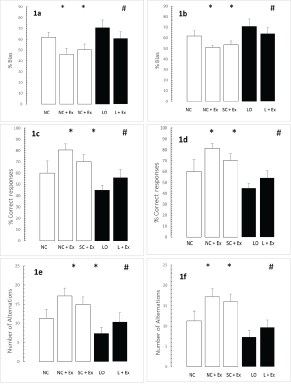
The effects of the swimming Ex on the exploration phase of passive-avoidance testing are shown in [Table/Fig-2a-d]. The swimming Ex resulted in no significant changes in the time spent within the smaller compartment [Table/Fig-2a,b] and in the number of crossings [Table/Fig-2c,d]. This pattern of behaviour was observed in the animals subjected to the Ex intervention one day after grouping in the normal and sham-operated controls and one day and after KA lesioning in the experimental rats [Table/Fig-2a,c], as well as 60 days after grouping in the normal and sham-operated controls and 60 days after KA lesioning in the experimental rats [Table/Fig-2b,d].
Effects of 30 d swimming Ex on exploration phase of passive-avoidance test. White bars compare means ± SD of time spent in the small compartment, expressed in s/trial) and total number of crossings in groups of 4 month-old male Wistar rats exposed to the following conditions: NC, NC+EX and SC+EX treatments. Black bars compare means ± SD of time spent in the small compartment and total number of crossings in groups of 4 month-old rats subjected to LO followed by L+EX. Panels a,b): Time spent in the small compartment; c,d): total number of crossings. a,c)-1 d post grouping for controls, 1 day postictal for lesioned rats; b,d): 60 d post grouping for controls, 60 d postictal for lesioned rats. Intergroup differences were not significant, p>0.1.
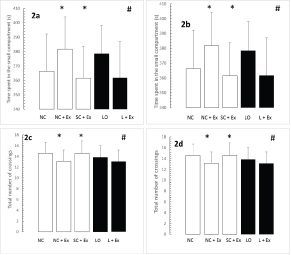
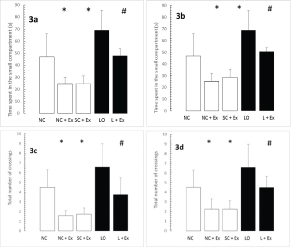
The effects of the swimming Ex on the retention phase of passive-avoidance testing are shown in [Table/Fig-3a-d]. The swimming Ex resulted in significant decrements in the time spent within the smaller compartment [Table/Fig-3a,b] and in the number of crossings [Table/Fig-3c,d]. These increments were observed in the animals subjected to the Ex intervention one day after grouping in the normal and sham-operated controls and one day after KA lesioning in the experimental rats [Table/Fig-3a,c], as well as 60 days after grouping in the normal and sham-operated controls and 60 days after KA lesioning in the experimental rats [Table/Fig-3b,d].
Effects of 30 d swimming exercise on memory in passive-avoidance test. White bars compare means ± SD of time spent in the small compartment with previous exposure to an aversive stimulus and total number of crossings in groups of four-month-old male Wistar rats exposed to the following conditions: NC, NC+EX and SC+EX treatments. Black bars compare means ± SD of time spent in the small compartment and total number of crossings in groups of four-month-old rats subjected to LO followed by L+EX. Panel a,b: Time spent in the small compartment; c.d): total number of crossings. a,c)-1 d post grouping for controls, 1 day postictal for lesioned rats; b,d): 60 d post grouping for controls, 60 d postictal for lesioned rats. *Different than NC at p<0.05; #different than LO at p<0.001.
Discussion
The main finding of this study is that aerobic (swimming) Ex attenuates the learning and memory deficits following chemically-induced (kainate) seizures, in a rat model of temporal lobe epilepsy. This Ex-induced augmentation of learning and memory occurred whether the Ex treatment was initiated one day or 60 days, postictal. The finding that this swimming Ex improves learning and memory is bolstered by our control studies in which the same swimming Ex regimen improved learning and memory in normal and in sham-operated control animals. This is the first study to demonstrate that swimming Ex attenuates learning and memory deficits associated with kainate-lesioning over the long-term, after a postictal delay in initiating the Ex treatment. We defined improvement in learning as a low percent bias, high number of alternations and high percentage of correct responses associated with a T-maze task. The Ex treatment was found to decrease the percent bias, increase the number of alternations and increase the percentage of correct responses in both the normal and sham-operated controls and in the kainate-lesioned animals. We defined improvement in memory as less time spent in the (small) compartment where the rat was previously subjected to an aversive stimulus and as fewer number of crossings between the compartments on a passive-avoidance task. The Ex treatment was found to decrease the amount of time spent in the small compartment and the number of crossings in the normal and sham control rats, as well as in the kainate-lesioned rats.
A study from our laboratory, using the same experimental design as that of the present study, demonstrates that swimming Ex is an effective stimulus to neurogenesis in the rat KA model of temporal lobe epilepsy [15]. The present study corroborates that the neurogenesis associated with such Ex may be positively correlated with improved cognitive functions, as previously reported [18]. Neurogenesis has mostly been studied in the neural progenitor (stem) cells within the subgranular zone of the hippocampal dentate gyrus in adult rats [19]. Neurogenesis is also known to occur in areas CA1 and CA3 of hippocampus. Brain-Derived Neutritrophic Factor (BDNF) upregulates neuronal dendrite growth and production of postsynaptic density protein-95, thereby reorganizing synapses (synaptogenesis) and promoting neural plasticity [15]. It is well established that regular Ex can enhance brain functions, including cognitive abilities and upregulate neurotrophins [15] and upregulation of neurotrophins has been reported following swimming Ex in rats, correlated with improved brain functions [15].
Exercise and Learning in Normal and Sham-Operated Control Rats
We measured percent bias, number of alternations and percentage of correct responses on a T-maze task, as well as time spent in the compartment where the animal was previously subjected to an aversive stimulus and number of crossings between the compartments on a passive-avoidance task. The measurements were made in separate groups of control animals at two time points immediately after grouping or sham operation, as well as 60 days after grouping or sham operation. It was beyond the scope of this study to determine the precise mechanism for the learning and memory improvements observed but, in accordance with previous research cited above, it is likely that the swimming Ex increased the production of neurotrophic factors in the brain areas studied [15]. The findings in our normal control and sham-operated rats demonstrate that the percent bias was decreased, correct responses were increased, the number of alternations was increased on the T-maze task and the time spent in the small compartment was decreased, the number of crossings was decreased on the passive-avoidance task. These results were found after exposure to the swimming Ex whether or not the Ex treatment was administered immediately or after a delay of 60 days following grouping or sham surgery. The purpose of studying this delay was to provide control data for a second experiment in which we evaluated the effects of the same intensity and duration of swimming Ex initiated in the immediate one day and delayed 60 days postictal periods on kainate-treated rats of the same strain, age and sex as the controls [15].
Exercise and Learning in Kainate-Lesioned Rats
We compared the data on the various components of the T-maze and passive-avoidance tasks after administering KA and observing seizures with and without the same 30 days swimming Ex treatment in the same manner as in the previous control experiments. Effects of the swimming Ex treatments were also compared for groups of rats subjected to the swimming Ex regimen in the immediate one day and delayed 60 days postictal periods. Our measurements of percentage of correct responses on the T-maze task and the time spent in the small compartment on the passive-avoidance task, as well as the other components of these tasks, support the hypothesis that the swimming Ex may have increased the number of hippocampal neurons surviving the kainate lesioning and the number of dendritic arborizations available for synaptogenesis [20]. The number of surviving neurons is reported to be correlated with the learning and short-term memory deficits observed in kainate-lesioned rats [21,22]. Therefore, the most likely explanation for our findings is that following the kainate-lesioning and seizures, there was a significant increase in neurogenesis, mediating the learning and memory improvements whether the Ex was initiated and completed within the first 30 postictal days or after a delay of 60 postictal days. This finding is significant because although aerobic Ex is known to augment neurogenesis in rats, humans incapacitated by temporal lobe epilepsy may be unwilling or unable to Ex and to maintain regular Ex regimens over the long-term.
Limitation
The main limitation of this study is that we employed manual methods for recording our data and did not have access to computer-based learning and memory tasks. A radial-maze rather than a T-maze, apparatus or a water-maze (Morris water maze), rather than two-compartmental, apparatuse for passive avoidance testing would have permitted automated, electronic recording of the data. The aforementioned computer-based methods might have made our results more robust, but are unlikely to have changed the interpretation. It was beyond the scope of this study to evaluate the seizure frequency during the experimental period. However, the swimming Ex was not conducted in a stressful manner, such as requiring the rats to swim without stop. Such an Ex would, no doubt, be stressful and could therefore increase the frequency of seizures.
Conclusion
The results of this study demonstrate that swimming Ex attenuates the learning and memory deficits in rats subjected to kainate-induced seizures, whether or not there is a 60 days delay in initiating the Ex treatment. Improvements in learning and memory in the rats subjected to the 60 days delay was found to be significantly less than those in the animals that were given the Ex treatment immediately, beginning one day after the kainate-lesioning and seizures. Previous studies demonstrate correlation between learning/memory deficits and neuronal loss in hippocampus associated with kainate-lesioning and temporal lobe seizures. These findings imply that aerobic Ex improves neural plasticity in hippocampus by increasing neurogenesis and synaptogenesis, with improvements in learning and memory even after a substantial delay.