Severe Left Ventricular Endomyocardial Fibrosis Presenting as Biventricular Failure in a Young Adult: A Case Report
Harpreet Singh Sandhu1, Sampathkumar Mahadevappa Mahendrakar2, Rajebali Ramzanali Pethani3, Azizullah Hafizullah Khan4, Yunus Shafi Loya5
1 FNB Critcal Care Medicine Trainee, Department of Intensive Care Medicine, Prince Aly Khan Hospital, Nesbit Road, Mazgaon, Mumbai, Maharashtra, India.
2 Intensivist, Department of Intensive Care, Prince Aly Khan Hospital, Nesbit Road, Mazgaon, Mumbai, Maharashtra, India.
3 Consulting Physician, Department of Medicine, Prince Aly Khan Hospital, Nesbit Road, Mazgaon, Mumbai, Maharashtra, India.
4 ICU Director, Department of Intensive Care, Prince Aly Khan Hospital, Nesbit Road, Mazgaon, Mumbai, Maharashtra, India.
5 Consulting Cardiologist, Department of Cardiology, Prince Aly Khan Hospital, Nesbit Road, Mazgaon, Mumbai, Maharashtra, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Harpreet Singh Sandhu, Anaesthesia and Critical Care, FNB Critical Care Medicine Trainee, Department of Intensive Care, Prince Aly Khan Hospital, Nesbit Road, Mazgaon, Mumbai-400010, Maharashtra, India.
E-mail: itallionstallion17@gmail.com
Endomyocardial Fibrosis (EMF) is a form of progressive restrictive cardiomyopathy of unclear aetiology prevalent in areas within 150 of equator including coastal areas of Kerala a few decades back. It inflicts young adults and carries a poor prognosis due to limited options for treatment. Fortunately, the incidence of cases is now declining due to improvement in health and hygiene standards. Here, we review the aetiology and pathogenesis of EMF and report a case of a young male from Mumbai (non-endemic area) presenting with progressively worsening breathlessness and signs of heart failure unresponsive to conventional medical treatment. To delineate the extent of the disease transthoracic echocardiography and cardiac Magnetic Resonance Imaging (MRI) was done which revealed infiltrative lesions in left ventricular apex with grade 2/3 mitral regurgitation. Due to progressive and severe nature of the disease the patient was managed conservatively. Through this report we would like to rekindle the interest of reader in a forgotten tropical disease which is considered rare in this geographical area but should not be missed as a cause heart failure considering its significant mortality.
Eosinophilia, Tropical disease, Heart failure
Case Report
A 35-year-old male patient resident of Mumbai (India) presented to the Intensive Care Unit (ICU) with body aches, weakness and progressive dyspnea worsening over last one week. His dyspnea had insidious onset progressing from New York Heart Association Grade II from past 6 to 8 months to dyspnea at rest on admission. There was no history of fever, weight loss, joint pains, burning micturition and sore throat. He was earlier evaluated on out-patient basis for his dyspnea and a transthoracic echocardiography (ECHO) was done six months back suggesting a mild Mitral Regurgitation (MR), of probable rheumatic origin. Patient was started on diuretics, beta blockers showing initial symptomatic improvement.
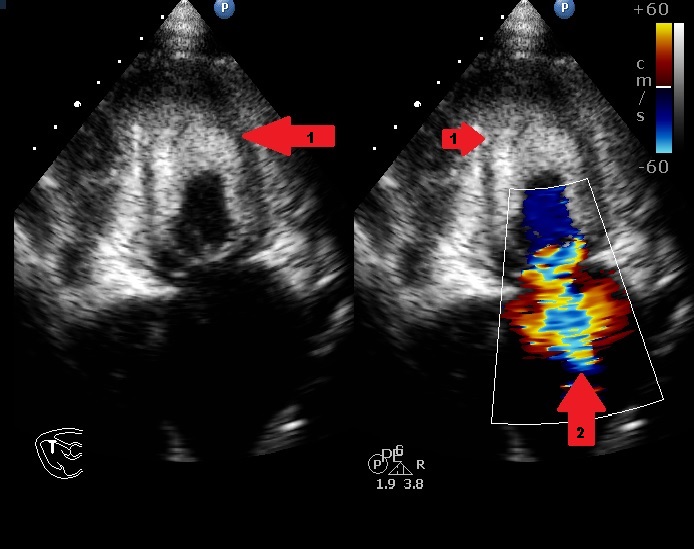
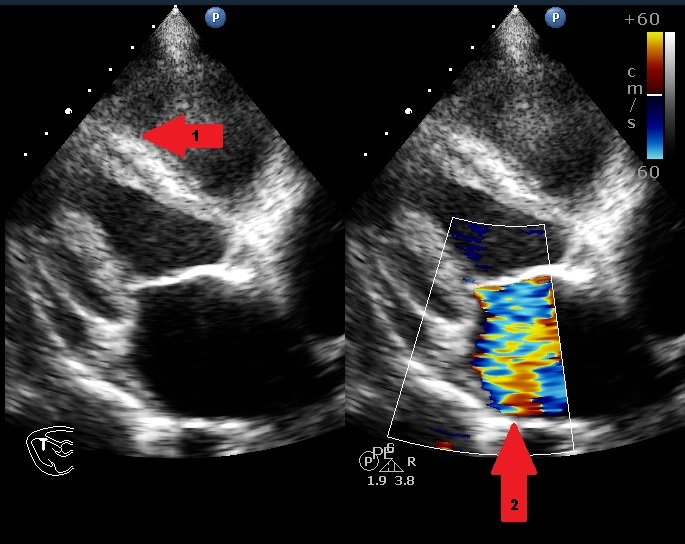
He had a Body Mass Index (BMI) of 23.89 kg/m2 and clinical examination revealed he was tachypneic, orthopneic with bilateral basal crepitations. Supplementary oxygen at 8 litres/minute flow was applied with facemask. Distended neck veins, a S3 gallop and oxygen saturation of 95% were noted. Abdomen was soft, non-tender and not distended. Electrocardiogram showed ST segment changes in inferior and lateral leads and chest roentgenogram showed pulmonary oedema. Bedside sonography done in semi-recumbent position revealed diffuse B-lines, more over basal lung fields suggestive of cardiogenic pulmonary oedema. Provisional diagnosis of heart failure was made and routine blood investigations were ordered showing normal haemoglobin, leucocyte counts with absence of eosinophilia. Serum troponin-I was <0.05ng/ml (normal<0.4ng/ml) and brain natriuretic peptide was 519pg/ml (normal<100pg/ml). Bedside ECHO revealed an infiltrative lesion in the apex of Left Ventricle (LV) suggestive of Endomyocardial Fibrosis (EMF) with preserved LV ejection fraction of 65%, mid LV peak gradient of 35mmHg, grade 2/3 mitral regurgitation (MR) and pulmonary artery systolic pressure of 70mmHg [Table/Fig-1,2] [Video-1,2]. Non-invasive ventilation, loop diuretics and beta blockers were initiated as the initial management strategy. In further workup serum anti-toxoplasma Immunoglobulin G (IgG) levels were found to be 87.0 using fully automated chemiluminescence system (titres >8 positive) while anti-toxoplasma immunoglobulin M (IgM) levels were negative. A Cardiac Magnetic Resonance Imaging (CMRI) was performed which showed diffuse arrow shaped sub endocardial enhancement along with the abnormal soft tissue along the mid and apical septum, lateral and inferior walls of left ventricle characteristic of EMF [Table/Fig-3,4 and 5] [Video-3,4]. Patient was started on intravenous high dose corticosteroids. His condition gradually improved over next few days. On the 10th day of admission, patient developed sudden Ventricular Fibrillation (VF) requiring Cardio Pulmonary Resuscitation (CPR) along with vasopressor support and mechanical ventilation. Patient improved clinically over the next 48 hours with stable haemodynamics. On day 14, he developed sudden and refractory VF for which high quality CPR was given. Unfortunately, early and aggressive resuscitation could not revive the patient.
Apical four chamber view on transthoracic ECHO showing apical fibrosis (arrow 1) and MR jet (arrow2)
Parasternal long axis view on transthoracic ECHO showing fibrosed papillary muscle, interventricular septum and left ventricle (arrow 1) and dilated left atrium with MR jet (arrow2).
CMRI four chamber view showing apical left ventricular endomycardial fibrosis (arrow 1) and MR jet as black shadow at mitral valve (arrow 2)
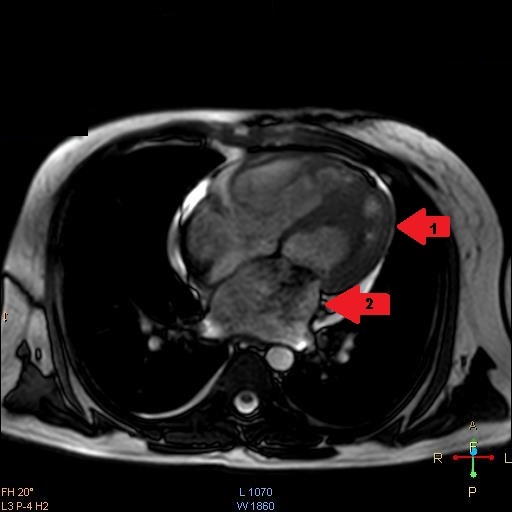
CMRI imaging two chamber view showing apical fibrosis compromising LV filling(arrow 1) and dilated LA with MR jet as black shadow at mitral valve(arrow 2)
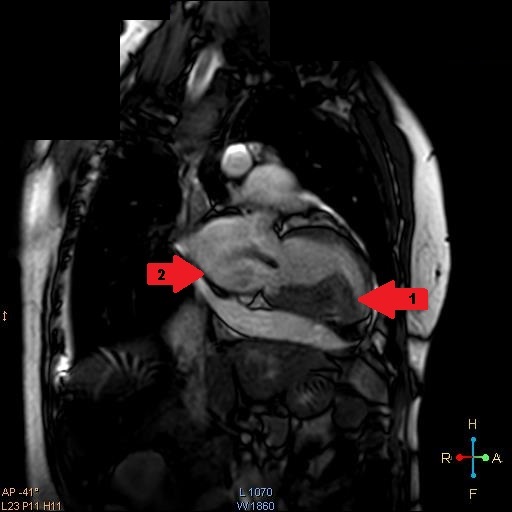
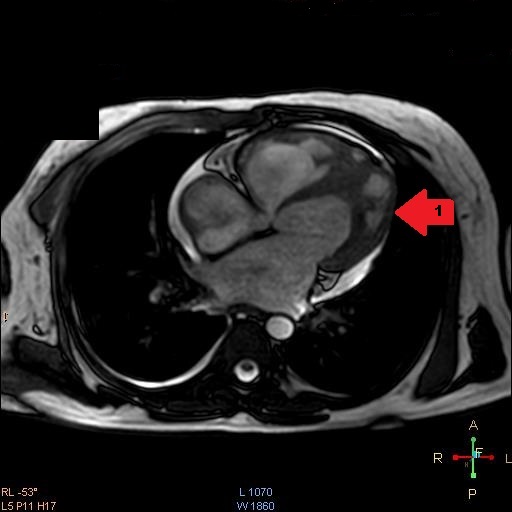
CMRI four chamber view during end systole view demonstrating apical LV fibrosis (arrow 1).
Discussion
EMF is a form of progressive restrictive cardiomyopathy which was first described by Jack NP Davies [1]. It frequently affects older children and young adults with most reported cases within 150 of the equator [2]. EMF was once an epidemic with over 2400 cases worldwide with half of the cases reported from sub-saharan Africa and quarter of cases being reported from Uganda alone [3]. The disease has an unexplained aetiology even after seven decades, with infection (filaria, malaria, schistosoma and toxoplasma) as the probable cause apart from environmental and dietary factors [1,4,5]. Toxoplasma gondii is an obligate intracellular parasite with a wide geographic distribution spread by eating undercooked meat and to children from infected mothers. In immunocompetent hosts it goes unnoticed causing flu like symptoms and lymphadenopathy though severe myocarditis is a reported complication [6,7].
EMF is characterized by sub-endocardial fibrosis involving the apex and inflow tracts of one or both ventricles producing a restrictive filling defect. Progressive fibrosis of papillary muscle leads to valvular regurgitation and progressive atrial dilation. Right Ventricular (RV) EMF with signs of RV failure is a common presentation. Isolated LV-EMF is rare and presents as progressive dyspnea due to pulmonary congestion [2]. EMF is sometimes considered a late burn-out phase of Loffler’s endocarditis which starts with eosinophilic myocarditis (1 to 2 months) progressing to endocardial thickening with thrombus formation at 10 months and progressive fibrosis at more than 2 years of disease [8]. Overall prognosis of EMF is poor with 84%-95% mortality at 1 to 3 years due to progressive heart failure, arrhythmias [9]. Due to the improving health and hygiene standards incidence of EMF is fortunately declining in India and has even been described as a vanishing disease [10].
EMF though rarely reported from this geographic location still continues to be important form of restrictive cardiomyopathy carrying high morbidity and mortality. The city though located outside the 15° zone of endemicity has a tropical wet and dry climate. The presentation as progressive breathlessness with MR on initial ECHO could easily be confused with rheumatic mitral disease which is more prevalent in this geographic location. The clinical presentation as decompensated heart failure with extensive fibrosis of LV itself predicted the poor prognosis in this case [11]. CMRI proves a useful diagnostic tool delineating the extent of EMF especially in early stage of disease [2,12]. The treatment with steroids might help during initial eosinophilic phase of myocarditis but probably not in advanced fibrotic phase. In this case there was initial modest improvement in symptoms but the patient finally succumbed to the severe and progressive nature of the disease. The presence of high titers (>8times) of anti-toxoplasma IgG antibodies suggest infection by Toxoplasma gondii and similar high titers have been reported in patients with EMF [13,14]. A high incidence of cardiotropic infective agents has been found in surgically resected endomyocardium in EMF patients [15]. EMF in this case probably represents the burnout phase of sub clinical myocarditis due to toxoplasma infection though a causal relationship could not be established due to abbreviated and rapid downhill clinical course in this case. Further studies are thus required to prove this association.
Conclusion
Through this report we try to rekindle the interest in a forgotten tropical disease as a cause of heart failure and its relationship with toxoplasma infection also highlighting the use of CMRI for early diagnosis and differentiation from rheumatic heart disease in doubtful cases.
[1]. Grimaldi A, Mocumbi AO, Freers J, Lachaud M, Mirabel M, Ferreira B, Tropical endomyocardial fibrosis: Natural history, challenges, and perspectivesCirculation 2016 133:2503-15. [Google Scholar]
[2]. Mocumbi AOH, Falase AO, Recent advances in the epidemiology, diagnosis and treatment of endomyocardial fibrosis in AfricaHeart 2013 99:1481-87. [Google Scholar]
[3]. Verma VK, Zafar KS, Tropical endomyocardial fibrosis: an overviewInt J Res Med Sci 2014 2:1267-77. [Google Scholar]
[4]. Davies J, Spry CJ, Vijayaraghavan G, De Souza JA, A comparison of the clinical and cardiological features of endomyocardial disease in temperate and tropical regionsPostgrad Med J 1983 59:179-85. [Google Scholar]
[5]. Bukhman G, Ziegler J, Parry E, Endomyocardial fibrosis: still a mystery after 60 yearsPLoS Negl Trop Dis 2008 2:97 [Google Scholar]
[6]. Montoya JG, Jordan R, Lingamneni S, Berry GJ, Remington JS, Toxoplasmic myocarditis and polymyositis in patients with acute acquired toxoplasmosis diagnosed during lifeClin Infect Dis 1997 24:676-83. [Google Scholar]
[7]. Bousquet A, Bigaillon C, Dumitrescu N, Larréché S, Godreuil C, Mestiri R, Acute myocarditis in an immunocompetent young man: Don’t forget Toxoplasma gondiiInt J Cardiol 2016 214:358-59. [Google Scholar]
[8]. Olsen EG, Pathological aspects of endomyocardial fibrosisPostgrad Med J 1983 59:135-41. [Google Scholar]
[9]. Kushwaha SS, Fallon JT, Fuster V, Restrictive cardiomyopathyN Engl J Med 1997 336:267-76. [Google Scholar]
[10]. Vijayaraghavan G, Sivasankaran S, Tropical endomyocardial fibrosis in India: a vanishing disease!Indian J Med Res 2012 136:729-38. [Google Scholar]
[11]. Gupta PN, Valiathan MS, Balakrishnan KG, Kartha CC, Ghosh MK, Clinical course of endomyocardial fibrosisBr Heart J 1989 62:450-54. [Google Scholar]
[12]. Yoshida A, Ishibashi-Ueda H, Yamada N, Kanzaki H, Hasegawa T, Takahama H, Direct comparison of the diagnostic capability of cardiac magnetic resonance and endomyocardial biopsy in patients with heart failureEur J Heart Fail 2013 15:166-75. [Google Scholar]
[13]. Montoya JG, Laboratory diagnosis of Toxoplasma gondii infection and toxoplasmosisJ Infect Dis 2002 185:73-82. [Google Scholar]
[14]. Ijaola O, Falase AO, Distribution of antibodies against Coxsackie B viruses, arboviruses and Toxoplasma gondii among patients with endomyocardial fibrosis (EMF) compared with normal subjects from EMF endemic and non-endemic zones of NigeriaAfr J Med Med Sci 1990 19:93-103. [Google Scholar]
[15]. Iglezias SD, Benvenuti LA, Calabrese F, Salemi VMC, Silva AMG, Carturan E, Endomyocardial fibrosis: pathological and molecular findings of surgically resected ventricular endomyocardiumVirchows Arch 2008 453:233-41. [Google Scholar]